eISSN: 2373-6372


Clinical Paper Volume 10 Issue 6
Gastroentherology, ABC School of Medicine, Brazil
Correspondence: Ethel Zimberg Chehter, Faculdade de Medicina do ABC, Av. Lauro Gomes, 2000- Vila Sacadura Cabral, Santo André, São Paulo, Brazil, Tel (11) 999629456, Fax (11) 32572057
Received: November 29, 2019 | Published: December 20, 2019
Citation: Carmo APPD, Batlle AR, ZimbergChehter E. Gastric cancer: A 40year review of clinical and histologicalfeatures of 1838 cases in the ABC region in São Paulo, Brazil. GastroenterolHepatol Open Access. 2019;10(6):312-317 DOI: 10.15406/ghoa.2019.10.00402
Introduction: Gastric cancer (GC) is the fifth most common type of malignant tumor, and it is the second main cause of cancer-related deaths worldwide. However, its epidemiology is not well known in Brazil, particularly in the ABC region in São Paulo despite its importance as an economic and industrial center.
Objectives: The aims of this study were to identify the patients' profile, to verify the possible patterns of change throughout the decades and in patients according to each age group, and to analyze the main anatomopathological characteristics of these neoplasias in patients who underwent gastric cancer surgery in the ABC region in São Paulo.
Methods: This is a retrospective review of 1,838 histopathological gastric cancer cases diagnosed at the Department of Pathology, Centro Universitário Saúde ABC between the years 1974 and 2014. Morphological characteristics, such as the type of neoplasia (Lauren's classification), the gastric region as well as the characteristics and stage of the disease were analyzed. The study was divided into 2 groups: patients separated according to their age group (over and below 60years of age) and according to the 4 studied decades.
Results: Throughout the 4 decades, there was a male predominance of gastric cancer in patients who were aged 50years and over (77.3%) with no other epidemiological differences. The most frequent neoplasia was adenocarcinoma and the diffuse type was the most common. According to Borrman's classification, type III was the most prevalent. Regarding the location, the gastric antrum was the most common region. Upon using the age-group parameter (over and below 60years), it could be observed that the most frequent neoplasia in both groups was adenocarcinoma. Regarding the region of the tumor and Borrman's classification, the gastric antrum and type III were respectively the most prevalent in all patients
Keywords: lauren, gastric cancer, histology, epidemiology, review
Despite the global incidence decrease over the past 6 decades, gastric cancer (GC) is the fifth most common type of malignant tumor, and it is the second main cause of cancer-related deaths worldwide. In the United States, although gastric cancer incidence rate has decreased 4times since 1930 and mortality rate has dropped by 86% since 1950, the disease still constitutes the seventh major cause of cancer death in the country. According to GLOBOCAN 2012, an estimated 950,000 new stomach cancer cases and 720,000 related deaths have occurred in the world since 2018. 1
The most recent epidemiological data show that there has been a steep drop in incidence of gastric cancer in developed countries as well as changes in the histopathological pattern of the disease,2–4 whereas in developing countries this number increases. Actually, more than 60% of the new cases are found in these countries. In spite of the different incidence rates and the differences in early detection programs between western and eastern countries, survival rate in five years is of approximately 30% in developed countries and 20% in developing countries.
Gastric cancer affects more males than females (2:1) both in incidence and mortality rates.5 It usually appears after 50years of age and incidence peaks when patients are in their 70s. In the United States, for example, the mean age at diagnosis is 71years whereas in Japan the mean age falls to 61years due to the numerous screening programs in this country. Among men, gastric carcinoma is the fourth most common form after colorectal, prostate and lung cancer. In women, it figures as the seventh most common neoplasia after corpus uteri, thyroid, cervix, lung, colorectal and breast cancer.
Socioeconomic status is also an important factor regarding the incidence of this pathology. Some studies reveal that the lower the status, the higher the incidence of the disease, a fact that may be associated with environmental and dietary factors. Genetic factors have also shown to play an important role in the onset of the disease.6
Many studies have reported that dietary factors are highlighted as being one of the major causes related to the onset of stomach cancer. High salt intake has been associated with higher rates of atrophic gastritis in humans, which leads to an almost two-fold higher risk of developing the disease. Other environmental factors, such as bad food conservation and the consumption of water from wells, are also related to the incidence of this neoplasia. Actually, this aspect has been deeply researched due to the fact some studies show that nitrosamides (carcinogenic substances for the gastric mucosa) can be formed in the human stomach through the interaction of nitrites and nitrates with other gastric contents.7
Helicobacter pylori is a microaerophilic gram negative bacteria that affects almost half of the world population. It is the main cause of gastric inflammation, and it is the main etiologic agent of gastric cancer. The infection by this agent leads to 3 possible outcomes: (1) superficial gastritis, with asymptomatic patients; (2) duodenal ulcer phenotype, which occurs in 10-15% of the infected patients; or (3) gastric cancer phenotype/gastric ulcer. It is important to point out that the increased risk of developing gastric adenocarcinoma caused by H. pylori infection depends on some factors like H. pylori strain, the host's genetic factor, the infection duration and the presence or absence of environmental factors mentioned above. Just like dietary habits and H. pylori infection, smoking is also a very important factor for the development of the disease.8 Extensive cohort studies carried out in Europe and Asia show that smokers are 1.5-fold more likely to develop stomach cancer in both cardia and non-cardia regions than non-smokers. While intestinal gastric cancer is associated with environmental factors like the ones mentioned above, diffuse gastric cancer is considered a malignant neoplasia recognized as primarily genetic. Recent breakthroughs in molecular genetics have shown that the accumulation of these abnormalities, including the activation of oncogenesis and the inactivation of tumor suppressor genes result in the development of cancer.9 Genetic alterations described for gastric carcinoma include amplifications and mutations of the c-ERBB2, KRAS, c-MET and TP53 genes. The gain of chromosomes has also occurred in many combinations with the loss of other chromosomes, and it may be associated with the high expression of oncogenes, which contribute to tumor progression.
Adenocarcinomas (malignant neoplasias that originate in glandular tissue) is responsible for 90-95% of all malignant neoplasias followed by lymphomas.10 In 1965, Lauren classified gastric adenocarcinoma into 2 histological types: intestinal and diffuse. The risk of the intestinal type is higher in men of advanced age, and it is more frequently found in the proximal than in the distal region. Injuries of the kind are more frequent, differentiated, environment-dependent and associated with the presence of pre-cancer lesions such as chronic gastritis, intestinal metaplasia (substitution of gastric epithelium for histological elements that reproduce duodenal epithelium) and dysplasia.
The diffuse type is more frequently found in people below 50years of age, and the odds ratio between men and women is close to 1:1. Poorly differentiated and with a bad prognosis, it usually presents in the form of large tumors with higher degree of penetration into the gastric wall. Besides, it is not associated with pre-cancer lesions.8,11,12 Gastric cancer behavior in Brazil is not known. Therefore, focusing on the ABC region in São Paulo, the current study aims to identify the profile of the population affected by the disease, to analyze the pathology behavior and its possible changes throughout the studied period, and to get to know the main anatomopathological characteristics of these neoplasias. Its histological pattern in the ABC region over the past 4 decades will be shown below.
This is a review based on 1,838 reports on primary gastric cancer cases diagnosed between January 1973 and December 2014 referred and analyzed by the Department of Pathology of Faculdade de Medicina do ABC, now Centro Universitário de Saúde ABC.
For each gastric cancer report, the following was considered: staging (Lauren's classification), affected gastric region, histological type, gastritis-affected area (if present)-and Bormann's classification. Additionally, epidemiological characteristics of the studied population, such as sex and age, were also taken into account. Patients were divided into 2 groups, namely, according to their age group (over and below 60years of age) and according to the 4 studied decades: group I (1973-1982), group II (1983-1992), group III (1993-2002) and group IV (2003-2014). All the reports were made by members from the Department of Pathology of the Faculdade de Medicina do ABC, Centro Universitário Saúde ABC -Brazil. This study only shows results that used Lauren's classification in the studied series. It was not possible to evaluate all the variables in all cases due to problems inherent to the samples themselves, tumor sizes, or even to the fact that some variables were not considered when the service was implemented. In order to analyze each proposed variable (gender, age…), they were related to the total number of cases of each variable in which the analysis was made possible. To categorize these variables, a descriptive statistical analysis was carried out.
In the current study, the mean age found was 62.2years (±9.6), and gastric cancer was more prevalent in men (68.8%), with a male to female ratio of 2.2:1. The most common type of cancer found was adenocarcinoma (94.3%) followed by carcinoma (3.9%) and other neoplasias (1.8%). There were no differences in age or sex distribution regarding the anatomical location or histological subtypes.
According to the subtype, the most frequently reported was diffuse type (50.7%). Nevertheless, the difference above intestinal type wasn’t very apparent. In the group under 45years, a total of 39 patients (11.6%) were identified as females with the diffuse type in relation to the total of the series.
In a pilot study of our group, a table was drawn up in order to correlate Lauren to gender, sex and other factors. Figure 1 shows the prevalence between the histological types of adenocarcinoma throughout the work. In the current study, the most common affected region was the gastric antrum (47.3%) followed by the gastric corpus (30.3%). Table 1 shows the analysis of variables carried out according to each decade. Except for the variable "tumor region", no major differences between variables could be observed.
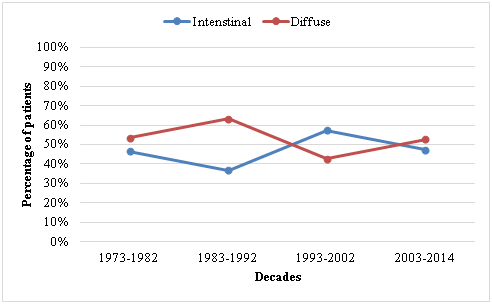
Figure 1 Prevalence according to Lauren's classification during the four decades of the study period.
Variables |
Group1 |
Group2 |
Group3 |
Group4 |
Total |
AGE |
N=124 |
N=223 |
N=638 |
N=853 |
N=1838 |
m±dp |
59.8±11.3 |
59.9 ±13.8 |
62.5±12.9 |
63±4.1 |
62,2 +- 9.6 |
Min-Max |
34-91 |
10-85 |
21-93 |
15-97 |
10-97 |
Neoplasm |
N=124 |
N=223 |
N=638 |
N=850 |
N=1835 |
Adenocarcinoma |
108(87.1%) |
201(90.1%) |
586(91.9%) |
834(98.1%) |
1729(94.3%) |
Carcinoma |
9(7.3%) |
12(5.4%) |
36(5.6%) |
14(1.6%) |
71(3.9%) |
Lymphoma |
2(1.6%) |
1(0.5%) |
6(0.9%) |
- |
9(0.4%) |
Others |
5(4%) |
5(2.2%) |
5(0.8%) |
- |
15(0.8%) |
Notdefined |
- |
4(1.8%) |
5(0.8%) |
2(0.3%) |
11(0.6%) |
Laurenclass |
N=43 |
N=98 |
N=350 |
N=793 |
N= 1284 |
Intestinal |
20(46.5%) |
36(36.7%) |
201(57.4%) |
375(47,3%) |
632(49,2%) |
Diffuse |
23(53.5%) |
62(63.3%) |
149(42.6%) |
418(52,7%) |
652(50,7%) |
Tumor region |
N=97 |
N=114 |
N=190 |
N=352 |
N=753 |
Cardia |
6(6.2%) |
9(7.8%) |
19(10%) |
26(7.4%) |
60(8.0%) |
Body |
25(25.8%) |
32(28.1%) |
59(31.2%) |
112(31.8%) |
228(30.3%) |
Fundus |
3(3.1%) |
1(0.9%) |
5(2.6%) |
7(1.9%) |
16(2.1%) |
Antrum |
59(60.8%) |
70(61.4%) |
96(50.5%) |
131(37.3%) |
356(47.3%) |
Others |
4(4.1%) |
2(1.8%) |
11(5.7%) |
76(21.6%) |
93(12.3%) |
Borrmann |
N=53 |
N=75 |
N=216 |
N=130 |
N=474 |
1 |
5(9.4%) |
2(2.7%) |
7(3.2%) |
7(5.3%) |
21(4.5%) |
2 |
15(28.3%) |
7(9.3%) |
72(33.3%) |
26(20%) |
120(25.3%) |
3 |
31(58.5%) |
65(86.7%) |
116(53.8%) |
81(62.3%) |
293(61.8%) |
4 |
2(3.8%) |
1(1.3%) |
21(9.7%) |
16(2.4%) |
40(8.4%) |
Table 1 The analyzed variables according to patient groups in each decade of the study period
Figure 2 shows an expressive drop in tumor incidence in the gastric antrum region throughout the study period. Nevertheless, the region remains at the top of the list as the most frequently affected area. In parallel, a slightly progressive increased rate of tumors in the gastric corpus region can be observed.
When patients were separated in 2 groups according to age (≤60years and >60years), adenocarcinoma was more prevalent in both groups followed by carcinoma. In patients under 60, the diffuse type was of higher incidence (61%) whereas in the other group the intestinal type prevailed (55.3%) as seen in Figure 3 and Table 2.
Variables |
Less than 60 years |
Over 60 years |
|
Total |
|||||
Neoplasm |
N=651 |
N=989 |
|
N=1640 |
|||||
Adenocarcinoma |
603 |
(92.6%) |
940 |
(95.0%) |
|
1543(94.1%) |
|||
Carcinoma |
32 |
(4.9%) |
33 |
(3.3%) |
|
65(4.0%) |
|||
Lymphoma |
4 |
(0.7%) |
4 |
(0.4%) |
|
8(0.5%) |
|||
Others |
5 |
(0.8%) |
7 |
(0.7%) |
|
12(0.7%) |
|||
Notdefined |
7 |
(1.0%) |
5 |
(0.6%) |
|
12(0.7%) |
|||
Lauren’s classification |
N=455 |
N=703 |
|
N=1158 |
|||||
Intestinal |
177 |
(39%) |
389 |
(55.3%) |
|
566(48,9%) |
|||
Diffuse |
278 |
(61%) |
314 |
(44,7%) |
|
592(51,1%) |
|||
Tumor region |
N=282 |
N=427 |
|
N=709 |
|||||
Cardia |
20 |
(7.1%) |
37 |
(8.7%) |
|
57(8.1%) |
|||
Body |
79 |
(28.0%) |
137 |
(32.1%) |
|
216(30.5%) |
|||
Fundus |
6 |
(2.1%) |
10 |
(2.3%) |
|
16(2.2%) |
|||
Antrum |
146 |
(51.8%) |
186 |
(43.6%) |
|
332(46.8%) |
|||
Others |
31 |
(11.0%) |
57 |
(13.3%) |
|
88(12.4%) |
|||
N=186 |
N=251 |
|
N=437 |
||||||
1 |
7 |
(3.8%) |
12 |
(4.8%) |
|
19(4.3%) |
|||
2 |
48 |
(25.8%) |
66 |
(26.3%) |
|
114(26.2%) |
|||
3 |
115 |
(67.8%) |
153 |
(60.9%) |
|
268(61.3%) |
|||
4 |
16 |
(8.6%) |
20 |
(0.8%) |
|
36(8.2%) |
|||
Table 2 Studied variables in relation to patients over and below 60 years of age.
Upon analyzing the most highly affected stomach region, it was observed that there was a higher incidence in the antrum in both groups followed by the corpus as shown in Figure 4.
The results found that our study shows the ratio between affected males and females was 2:1 and the age group in which most cases were found was between 60 and 80years. No other significant epidemiological differences could be observed. This findings were similar to those of the study performed by John R. Kelley, in Austria.13
In our research, the main age was 62.2 and prevalence in men was 68.8%. Accordingly, in another study conducted in Rio Grande do Sul, Brazil the mean age was 62years, and 67.8% of the patients were men whereas 32.2% were women (2:1), also according to what we have observed.14
According to Lauren's classification, intestinal subtypes were found in 48.1% of the cases and the diffuse type in 40.9% and our research have seen that in currently period of 25years, there were no changes in the anatomical distribution; however, a significant decrease in the intestinal subtype and an increase in the diffuse subtype could be observed.
Our results shows that the diffuse type in patients under 60 was of 61%, while in patient over 60, 55.3% was of intestinal type, and fortunately, very similar to the results here found, in the study by E. Yakirevich, there was a prevalence of the intestinal type in patients over 50years, and in those under 50 the diffuse type was more prevalent.15 Interestingly, there was a diverging point between both works: the Lauren's classification. In the current study the difference between the intestinal and the diffuse types was never very expressive over the studied period, but the diffuse type was the most frequently reported (50.7%). On the other hand, in the study just mentioned, 54% of the patients presented with the intestinal type and 46% with the diffuse type.
In opposite of our research, a study conducted by C. de Martel,16–19 found out that 14.3% of the tumors were found in cardiac region, 3.3% in gastric fundus, 28.7% in corpus area, 15.2% in corpus and antrum regions and 38.5% only in antrum, while our data shows that the most prevalent area was antrum. It could also be observed that according to the Bormann's classification, type III was most commonly found (61.8%), and upon analyzing these two variables together, it can be concluded that the tumors were in an advanced stage when diagnoses were established.
The results here found suggest that the ABC region in São Paulo shows aspects similar to those found in the literature of developed countries regarding gastric cancer, maybe due to high life standard and development rates in the area. On the other hand, the great number of advanced stage tumors and their high incidence in the non-cardia region are signs of underdevelopment. Therefore, as there are characteristics of both sides of development, it is necessary to create a new approach to the disease so that an early diagnosis can be made and treatment can be more effective. Unfortunately, cases of late diagnosis of gastric cancer in this study were found at the department, a situation that will hopefully be reversed as soon as possible.
None.
The author declares no conflict of interest.
None.

©2019 Carmo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.