eISSN: 2373-6372


Review Article Volume 13 Issue 4
Department of Pharmacology, Medical and Clinical Studies Research Institute, Egypt
Correspondence: Manal ME Ahmed, Department of Pharmacology, Medical and Clinical Studies Research Institute, National Research Centre, Giza, Egypt, Tel 202(01093627027)
Received: August 10, 2022 | Published: August 27, 2022
Citation: Ahmed MME. Future approaches for Brucellosis vaccines and therapies development based on molecular host-pathogen interaction. Gastroenterol Hepatol Open Access. 2022;13(4):145-154. DOI: 10.15406/ghoa.2022.13.00514
This review discusses Brucella-host interactions on molecular base and brucellosis immunobiological response. Also, the review handles pathogenesis-informed rationales to prevent or treat brucellosis. Brucella species is an animal pathogen that may cause incidental human brucellosis as a zoonotic disease. Brucellosis results in worldwide economic losses, human morbidity, and poverty. Despite Brucella species infect humans as an incidental host, 500,000 new human brucellosis occur annually, and no approved human vaccines or patient-friendly therapies are available. Brucella has strong tissue tropism for lymphoreticular and reproductive systems with an intracellular lifestyle that hinders its exposure to innate and adaptive immune responses, sequesters the pathogen from antibiotics effect and drives clinical disease manifestations and pathology. Stealthy brucellae exploit strategies to establish infection, including i) evasion of intracellular destruction by restricting fusion of type IV secretion system(T4SS) dependent Brucella-containing vacuoles with lysosomal compartments, ii) inhibition of apoptosis of infected mononuclear cells, and iii) prevention of DC maturation, antigen presentation, and activation of naive T cells, pathogenesis lessons that may be informative for other intracellular pathogens. Data sets of NGS of Brucella and host time-series global expression fused with proteomics and metabolomics data from in vitro and in vivo experiments now approved human vaccines inform interactive cellular pathways and gene regulatory networks enabling full-scale systems biology analysis. The newly identified effector proteins of Brucella may represent novel targets for safer and more effective brucellosis vaccines and treatment.
Hippocrates on the Mediterranean littoral described brucellosis-like symptoms in humans. David Bruce and The-mistokles Zammit would discover Micrococcus melitensis, the brucellosis-causing agent, many centuries later, in 1886. They would understand that the brucellosis that affected the armed forces on Malta Island was caused by goat milk products. The zoonotic illness brucellosis is caused by the Brucella species, which are highly dangerous animal infections that cause significant economic losses in the livestock sector and widespread human morbidity.1 Numerous terrestrial and aquatic mammals, including cows, buffaloes, sheep, goats, pigs, dogs, dolphins, whales, seals, and desert wood rats, are susceptible to the Gram-negative bacterium known as Brucella species. There are six well-known species of Brucella, which are B. abortus, which infects cattle, B. melitensis, which infects sheep and goats, B. suis, which infects pigs, B. ovis, which infects sheep, B. canis, which infects dogs, and B. neotomae, which infects wood desert rats. B. Inopinata, a human; B. pinnipedialis and B. Ceti, aquatic mammals; and B. Microti, a common vole, are the other four recently discovered species. It's still unclear what the main host preference is with relation to the pseudogenes that influence host adaptation. The global burden of brucellosis among animals is substantial. Of the 1.4 billion cattle in the world, Brucella is thought to be present in more than 300million of them. In animals, brucellosis primarily causes abortion and other diverse symptoms. Brucella's accidental host is the human. Humans are frequently infected with Brucella by direct contact with the tissues or blood of sick animals or through consumption of contaminated animal products, like raw milk and unpasteurized cheeses. An estimated 500,000 new cases of brucellosis in people occur annually. High, fluctuating fever is a characteristic feature of acute human brucellosis. Arthritis, orchitis, hepatitis, encephalomyelitis, and endocarditis are among the various organ diseases that can develop from chronic brucellosis.2,3 Arthritis is the most frequent brucellosis side effect. The disease's numerous presentations make a clinical diagnosis more difficult. For more than a century, brucellosis has defied systematic efforts to eradicate it, even in the majority of affluent nations. Additionally, there is no licenced human vaccine on the market. Brucella species are class B diseases and potential bioterrorism agents due to the minimal number of pathogenic organisms needed for infection and their ability to aerosolize. The predicted financial risk of such an attack is second only to anthrax and tularemia with an infectious dosage of 10 to 100 organisms. Furthermore, the danger of intentional release creates a direct risk to public health in a population of urban dwellers that cannot be eliminated by the usual method of animal vaccination. Due to systematic domestic livestock screening and animal vaccination, brucellosis in humans and animals is generally uncommon in industrialised nations. But brucellosis is endemic in many poor nations, particularly in the Middle East, Asia, Africa, and South America, as well as in the United States, where disease foci continue to exist as a result of persistent infection in wildlife species. In order to help with the development of effective management for brucellosis, this review focuses on Brucellae host interactions and Brucella immunobiology as the keystone of its pathogenesis.
Brucella-host interactions
Brucellosis pathology
Brucella exhibits strong tissue tropism and consistently replicates in the vacuoles of dendritic cells (DCs), macrophages, and placental trophoblasts. Numerous mammalian cells, including microglia, fibroblasts, epithelial cells, and endothelial cells, can support Brucella reproduction. The intracellular lifestyle of Brucella minimises its exposure to the host’s innate and adaptive immune responses.4 The incubation phase, which occurs prior to the onset of clinical symptoms, the acute phase, during which the pathogen invades and disseminates in host tissue, and the chronic phase, which can eventually result in severe organ damage and host death, are the three distinct phases of Brucella infection. Non-specific flu-like symptoms in people can include pyrexia, diaphoresis, lethargy, anorexia, myalgia, and arthralgia. Furthermore, mounting evidence from endemic regions suggests that exposure is linked to a higher incidence of human abortion.5 Chronic infection results from the pathogen's capacity to survive in the host cells. In which brucellae are dispersed through the lymphoreticular system and ultimately result in cardiovascular, hepatic, lymphoreticular, neurologic, and osteoarticular illness. Measurable splenomegaly is accompanied by significant increases in the percentage of splenic macrophages, a modest decrease in the percentage of splenic CD4 and CD8 T cells, and an increase in the lymphohistiocytic cells in the spleen.
Brucella biological characteristics
In living organisms, Brucellae move quickly over the mucosal epithelial layer6 and are ingested by dendritic cells and mucosal macrophages. The pathogens are able to replicate and survive inside specialised phagocytic cells, escape the host’s immune system by altering it, and spread to their preferred tissues through cellular tropism, such as the reproductive tract, foetal lung, reticuloendothelial system, and placental trophoblasts in pregnant women.7 The adhesion, internalisation, intracellular trafficking, survival, and replication of Brucella in vulnerable hosts have all been extensively studied in vitro as models. Brucella therefore generates a zipper-like mechanism for internalisation after pathogen attachment to the surface of mucosal epithelial cells.8 Brucella bind to epithelial cell surface receptors that include sialic acid and sulfated residues before and/or upon contact with epithelial cells via activating as-yet-incompletely characterised binding molecule(s).9 The process of binding encourages the activation of small GTPases, which sets off a signalling cascade that rearranges the actin cytoskeleton and causes the host cell membrane to change along the pathogen's surface, enhancing invasion. Within a few minutes of contact, entry takes place, necessitating complete activation of a mitogen-activated protein kinase signalling pathway. 8 Brucella can replicate and survive for up to 72hours inside non-professional phagocytic cells in vitro, and it can pass epithelium in vivo by disrupting the function of the mucosal epithelial barrier.6 This connection simultaneously triggers a mild innate immune response with weak proinflammatory activity.8,10 Brucella are absorbed by mucosal phagocytic cells after being transported through the epithelium, and only 10% of phagocytized bacteria manage to adapt. However, some Toll-like receptors (TLRs; primarily TLR2, TLR4, and TLR9) initiate limited intracellular signalling that activates the transcription factor NF-kB to control expression of inflammatory cytokine genes, although at a level that is 10-fold less than enterobacteria.11 This allows Brucella to delay being recognised by the immune system and triggering an immune response.
The Brucella-containing vacuole (BCV), which is found inside mononuclear phagocytic cells, is where Brucella dwells, modifies intracellular trafficking, and transforms the vacuole into a replicative compartment or brucellosome.12 Experimental research demonstrates that Brucella quickly adjusts to the BCV’s restricted food supply microenvironment after invasion. In order to adjust to low oxygen tension, the pathogen initially suffers quantitatively reduced gene expression and protein synthesis associated with anabolic metabolism while boosting amino acid catabolism, moving to other energy sources, and changing respiration.13 Early after infection, a type IV secretion system (T4SS) was expressed in an in vitro brucellosis infection model. Inside mammalian cells, it is necessary for intracellular survival and cell division. The T4SS is required for sustained persistence even though in vivo studies have shown that it is not required for invasion, systemic dispersion, or the formation of initial infection.14 T4SS expression elicits an inflammatory response, which has been postulated as a method for attracting cells that support persistence.
Over the course of the infection, invading brucellae that survived the adaptation phase gradually regain the expression of important genes that are encoded for metabolic processes. The main targets of this transcription-translation reactivation are cell membranes, transporters, and iron metabolism.13 Virulence genes, which are sometimes also tightly controlled by quorum-sensing molecules, are among the essential activities that Brucella resume expression along with replication.15,16 After 12hours, which corresponds to the start of Brucella reproduction, infected mononuclear phagocytic cells recover to normal levels after experiencing considerable transcriptional alterations in response to infection during the adaption stage. Brucella has a number of cunning tactics to establish and maintain a chronic infection, including inhibition of apoptosis of infected mononuclear cells, prevention of dendritic cell maturation, reduced antigen presentation, and reduced activation of naive T cells, among the early transcription changes that contribute to adaptation.17 The ability of Brucella to adapt to the intramacrophage environment allows it to extend its intracellular persistence indefinitely, which aids in systemic metastasis and the infection of preferred target cells or tissues like placental trophoblasts, foetal lung, male genitalia, skeletal tissues, reticuloendothelial system, and endothelium. For a more comprehensive systems biology investigation of the pathogenesis of brucellosis at the level of the entire host organism, there is currently little evidence available to characterise the interaction of Brucella with these target cells and tissue.18,19
Traffick of Brucella inside cells
By inhibiting fusion of the BCV with the lysosomal compartment, Brucella causes intracellular damage. Within BCVs that have endoplasmic reticulum markers, some BCVs that host internalised Brucella go from endocytic compartments to a replicative niche (ER). The ER’s structural features and functional rearrangement are accompanied by BCV seizure of its membranes and constituent parts. Later,20 BCVs develop autophagic characteristics and show positive for lysosome- associated membrane protein-1, which is a distinguishing hallmark of the intracellular Brucella lifecycle (Figure 1-Figure 3). In order for an organism to establish an intracellular replicative niche in vitro, it must have the VirB T4SS, which controls Brucella intracellular trafficking.21,22 It is believed that the T4SS of Brucella secretes chemicals that regulate the pathogen's intracellular and covert lifestyle.23–25 Table 1 lists the recognised components of the host-pathogen interaction during the pathogenesis of brucellosis.
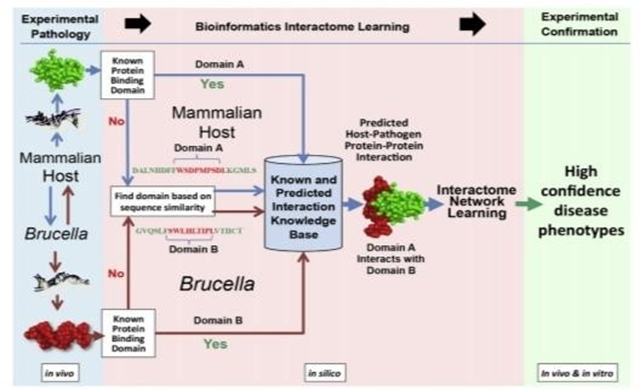
Figure 2 Scheme of in vivo and/or in vitro systems biology analysis of host and Brucella interactive pathogenesis.
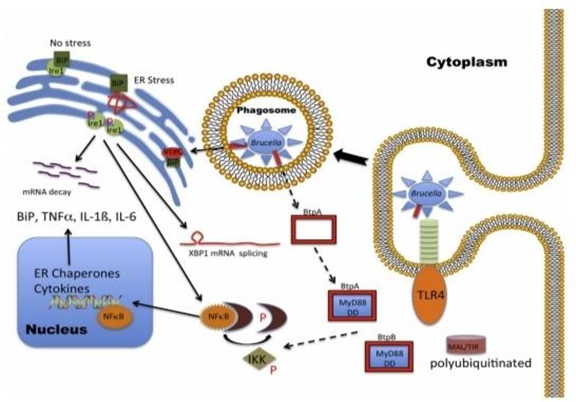
Figure 3 Brucella is bipolar. Brucella both inhibits and promotes a proinflammatory immune response.
Brucella element |
Host factor |
Reference |
Adhesion and internalization |
Sialic acid residues |
9 |
Intracellular trafficking |
GTPase Rab2 |
25 |
Intracellular survival |
Cholesterol |
28 |
Evasion of immunity |
MyD88 |
29,30 |
Proinflammatory reaction |
MyD88 |
24 |
Table 1 The established elements of the host-pathogen interaction during the pathogenesis of brucellosis
Plasma membrane-associated lipid rafts mediate the internalization of smooth Brucella into macrophage cells. As the BCV matures, it sequentially associates with markers for early (EEA1, purple circle; Rab5, blue diamond) and late (Rab7, orange square) endosomes. The biogenesis and trafficking of BCVs is regulated by bacterial effector proteins (white circles), which are secreted through the Brucella T4SS. BCVs that contain virulent organisms do not fuse with lysosomes (cathepsin D, gray trapezoid), although transient association with LAMP1-positive membranes (orange triangles) is observed. The pathogen replicates in tight rBCVs that are decorated with calreticulin (green triangle), a marker for the ER. At a later point after infection (48 to 72hours), the pathogen is observed in LAMP1-positive aBCVs that also contain LAMP1. The biogenesis of aBCVs depends on the activities of a subset of autophagy proteins, including ULK1 (not shown) and Beclin1 (not shown). Finally, the pathogen is released from the cell through lytic or nonlytic (shown) mechanisms. aBCV, autophagic Brucella-containing vacuole; BCV, Brucella-containing vacuole; Beclin1, coiled-coil myosin-like BCL2-interacting protein; EEA1, early endosome antigen 1; ER, endoplasmic reticulum; LAMP1, lysosome-associated membrane protein 1; rBCV, replicative Brucella-containing vacuole; T4SS, type IV secretion system; ULK1, Unc-51-like kinase 1.32
Regulatory systems
Regulatory systems play a crucial part in the stealth programme, which consists of two parts. The two-component BvrR/BvrS regulatory system and the LuxR-like transcriptional regulator VjbR are among the best investigated.16,33 The absence of the BvrR/BvrS sensory-regulatory system leads to a great changes in the outer membrane of bacteria which alters cellular uptake of the organism.34 The BvrR/BvrS regulon also regulates the expression of additional transcriptional regulators among 127 differentially regulated genes, in addition to carbon and nitrogen metabolism.33 The organism is avirulent in the mouse model and unable to replicate intracellularly in the absence of a functioning BvrR/BvrS. Ten transcriptional regulators, including vjbR, are involved in the regulation of the genes by BvrR/BvrS. It has been demonstrated that the vjbR protein controls the expression of the VirB locus, which encodes the T4SS required to prevent phagosome-lysosome fusion. 21 The reduced virulence of bvrR/bvrS mutants may be explained by the loss of VirB alone.
T4SS-Secreted Substrates identification
It has been discovered that knowing the Brucella virulence factors makes hosts more amenable to invasion and infection. The surface Opolysaccharide was the only place where the virulence components were present until the late 1990s.35 After that, numerous research groups employed diverse genetic strategies to inactivate target genes. These methods led to the conclusion that Brucella can stay in the host’s nutrient-poor BCVs, where it replicates and spreads infection with little involvement of the host cell.36,37 The T4SS stood out as a crucial target for additional exploration of virulence potential since it is reversible to the majority of metabolic activities discovered to be necessary for intracellular reproduction.38,39 Unfortunately, the exact mechanism of action has not yet been established. While the necessary defence against lysosome trafficking entails interacting partners, enzymatic processes, protein changes, and intricate intracellular trafficking. Following multiple investigations in which it was discovered that Brucella gene reporter fusions were released in a T4SS-dependent manner, these processes have just recently been reported.23,25,40 A number of factors, such as shared characteristics with effectors expressed by other bacteria, eukaryotic motifs, GC content, and restricted distribution across bacterial genera, were used to identify putative effector candidates in silico. When produced ectopically, candidate effectors were found to target secretory system compartments and inhibit host protein production.31,40 Genetic investigations have discovered functional redundancy among effector candidates, which is consistent with the failure of early genetic screens to find effectors. Many factors have been reported in the modern era. Although the list has not yet been fully compiled, it is still unknown how Brucella replication is boosted and lysosomal fusion is prevented, as well as the identity of effector targets and the enzymatic activity of effectors.
The Host unfolded protein response (UPR) role
A conserved evolutionary process called the host UPR mediates cellular adaptation to stress caused by protein folding in the endoplasmic reticulum (ER). When unfolded proteins build up in the ER lumen, the stress sensors activating transcription factor 6, protein kinase RNA-like ER kinase, and inositol-requiring enzyme 1 a (IRE1a), which are situated in the membrane of the ER, start the UPR process. Chaperones that aid in protein folding are controlled in expression by activating transcription factor 6. In situations of unresolved stress, protein kinase RNA-like ER kinase facilitates temporary translational attenuation and encourages apoptosis. When unfolded proteins build up in the ER, IRE1a plays a crucial function in triggering the UPR by accelerating the splicing of X box binding protein I (XBP1) mRNA. Following translation of the spliced message, active XBP1 transcription factor is produced. 41 XBP1 regulates the expression of UPR genes that produce proteins involved in ER-associated degradation, ER chaperones, and other proteins that lessen the negative effects of unfolded protein accumulation.42 For Brucella to live an intracellular lifestyle, the UPR must be subverted.22,43 IRE1a deletions in murine embryonic fibroblasts were used to show that this protein promotes Brucella intracellular replication in vitro. Brucella absorption was confirmed to be mediated by host phosphatidylinositol 3-kinase (PI3K) activity, however replication following uptake was unaffected. 43 In an ER-derived replicative BCV, Brucella replication takes place before the acquisition of autophagic markers, according to research by Celli and colleagues. 43 Unc-51-like kinase 1, Beclin 1, and other autophagy proteins are acquired as autophagic BCVs gradually emerge. ATG5 and LC3b are not required for the establishment of autophagic BCVs, which result in pathogen-containing compartments that are lysosome associated membrane protein-1 positive and calreticulin negative. Importantly, it was shown that autophagic BCVs were critical for the pathogen's ability to move from cell to cell.44 Finally, both in vitro and in vivo, Brucella infection causes XBP1 splicing and UPR gene expression in host macrophages.20 Additionally, tauroursodeoxycholic acid, a pharmacologic chaperone that lessens the UPR, is administered to host cells and inhibits Brucella replication.20 These findings collectively imply that Brucella manipulates host IRE1a signalling cascades to establish an intracellular environment that facilitates food uptake, pathogen multiplication, or pathogen cell-to-cell dissemination.
The role of host PI3K Activity and Brucella TIRAP-Containing Proteins
Particularly fascinating is the role of PI3K in Brucella uptake. Tolle IL-1 receptor (TIR)-containing proteins are encouraged to bind to the plasma membrane by PI3K, which is essential for the production of phosphatidylinositol3–5 trisphosphate and subsequent TLR signalling. In an effort to limit the proinflammatory response and dendritic cell (DC) maturation, Brucella express at least two TIR-containing proteins. 20,29,30,45,46 The mechanism’s specifics have yet to be determined. The presence of TIR-containing proteins TcpB, BtpA, and/or BtpB compete with MyD88 for binding to the TIR domain-containing adaptor protein (TIRAP), which leads to increased degradation of TIRAP by and interference with TLR4/TLR2 signalling.30 Phosphoinositide phosphate binding sites found in TcpB/BtpA and BtpB are consistent with binding at the plasma membrane and suppression of downstream processes that limit activation of NF-kB-mediated transcription and the emergence of an effective proinflammatory response.
Brucella and host omics analysis
Modern advances in next-generation sequencing (NGS), genomics, and omics technology allow for a thorough examination of Brucella pathogenesis. Proteomics and metabolomics data from in vitro and in vivo investigations on target species can be integrated with simultaneous Brucella and host global expression data sets on a large scale to create cellular pathway and gene regulation networks47–49 that allow for full-scale systems biology analysis.50 These technologies have helped us gain a better grasp of the entire Brucella pathogenesis system. The Brucella research community produced genomes and data sets during the turn of the century. 7,51,52 Brucella melitensis strain 16M has the first Brucella genome to be sequenced and published.53 More than 18 complete and 415 whole genome shotgun Brucella genomes have been sequenced, and they are all accessible for analysis online and published in peer-reviewed journals. The examination of comparative gene structure and homologies, conservation and variability, gene expression, regulatory networks, protein synthesis, interaction, and metabolic pathways may all be done using these databases. The construction of in silico inter- actome models of the infection biology of Brucella-host relationships will become increasingly easier as DNA sequences for the genomes of various host species, such as domestic animals and humans, are made available. These resources include experimental omics technologies, omics software tools, and omics technologies. By moving toward a more thorough examination of the host and Brucella species over the past ten years, various investigators have created in vitro and in vivo Brucella and/or host gene expression and proteome data sets.6,8,13,16,18,33,54-61 The identification of candidate genes and biomarkers of Brucella and hosts, the analysis and prediction of Brucella antigenic proteins, the development of subunit vaccines, the characterization of the severe Brucella stress response, the modulation of host responses, and other purposes have all made use of these massive data sets.54 In order to classify the connections of specific proteins within Brucella-host protein- protein networks, time-series analyses of experimental infection data collected in vitro or in vivo were carried out. On the basis of predicted protein-protein structural homologies, time-series studies of in vivo systems have been used as a source of Brucella gene expression data that have computationally interacted with bovine host gene expression data to identify mechanistic genes of interacting cellular pathways as novel biosignatures and potential druggable targets.62,66 Reverse vaccinology63 was used in conjunction with systems biology analysis of Brucella and bovine host gene expression data62,66 in a different time-series investigation to find promising candidate subunit vaccines that are protective against virulent challenge in the mouse model. 67 These studies offer persuasive evidence in favour of the use of systems biology to better integrate and utilise data for model development, causal discovery, biological activity prediction, improving the design of in vitro and in vivo experiments, finding biomarkers for improved brucellosis diagnosis, and finding druggable targets for more efficient brucellosis treatment
Experimental pathology includes the collection and omics (transcriptomics, proteomics, metabolomics, etc.) data from both mammalian host and Brucella samples from an in vivo time series of Brucella infection of a target organ (eg, Peyer patch, lung, spleen, liver) in a natural target animal (eg, cattle, sheep, goat, pig, nonhuman primate). The resulting omics (eg, transcriptomics, proteomics, metabolomics) data sets are fused and bioinformatically analyzed for known and computed structural modeling of predicted host- pathogen protein-protein interactions to develop an in silico interactome structure learning model. Proteins are inferred from genes if not directly measured. Pairs of predicted candidate Brucella-host protein-protein mechanistic genes from interactive pathways are phenotyped in vitro in standardized gentamicin killing assays by using specific deletion mutants of Brucella, siRNA knockdown of host genes, and confocal microscopy. Brucella-host protein-protein pairs with positive in vitro phenotypes are phenotyped in vivo, and high confidence positive candidate protein pairs undergo pull-down analysis and quantitative selected reaction monitoring mass spectrometry for further confirmation of Brucella-host protein-protein interactions in Brucella pathogenesis. Blue lines and arrows indicate flow of in vivo data and results from the host, and brown lines and arrows indicate flow of data and results from Brucella into in silico analysis to identify and model domain A from a host protein predicted to interact with domain B of Brucella.32
Brucellosis immunological response
Immunoregulatory components
The primary factor contributing to Brucella’s stealthy behaviour is the structure of the smooth lipopolysaccharide (LPS) on the cell surface.10 It was discovered that the elongated fatty acid molecules on the lipid A section reduced the toxicity of Brucella LPS and suppressed the immune response by acting as a subpar TLR4 agonist, consistent with Brucella’s ability to penetrate with little activation of the host cell. Rough Brucella species, on the other hand, lack the O-polysaccharide component of LPS, making them hazardous to macrophage cells. Although the lipid A of smooth and rough Brucella species has not been compared, it is not believed that the length of the fatty acids will change. Furthermore, a role for rough LPS in the cytotoxic activity is ruled out by the full elimination of cytotoxic activity in the absence of the T4SS. Currently, the most straightforward explanation is that, while O-polysaccharide is essential to the organism's stealthy behaviour, Brucella LPS is a weak TLR4 agonist. Weak TLR4 activation may be expected to inhibit uptake as seen with smooth Brucella because it is an inducer of PI3K activity, but higher uptake seen with rough Brucella is consistent with increased PI3K activity and a decrease in stealth.43 Brucella LPS also expresses unique immunoregulatory molecules that inhibit the innate immune response in addition to its poor agonist action. Some of these proteins, like the TIR-containing protein or Brucella TIR protein, TcpB/BtpA, have elaborated mechanisms.20 TcpB, like TLRs, has a TIR domain that it uses to communicate with cytoplasmic MyD88 adaptor-like/TIRAP. According to one study, MyD88 adaptor like/TIRAP degradation is sped up as a result of TcpB binding, which also disrupts TLR signalling and the production of pro- inflammatory cytokines.30,46 According to other studies, TcpB and MyD88 interact to block downstream connections and interrupt TLR-mediated activation.29 Although DtcpB Knockout mutants in the wild-type mouse model do not significantly alter survival, replication in macrophages is reduced 48hours after infection.20 Recent research also reveals that the lack of tcpB expression causes an enhanced level of immunological activation that lowers Brucella survival in general. One implication of this theory is that TcpB may function via protein kinase B to alleviate the suppression of target of rapamycin activity caused by the tuberous sclerosis complex 12 in order to ultimately reduce the NF-kB-mediated proinflammatory response and stimulate the production of IL-10. To do so, TcpB must increase the generation or stability of phosphatidylinositol-3,4,5 triphosphate, which would decrease the amounts of phosphatidylinositol 4,5- bisphosphate and weaken the connections across plasma membranes that promote TLR signalling. Currently, it is being investigated whether a second protein, BtpB, which has TIR structural domains, can block TLR signalling via MyD88 to stop DC from maturing.24 The failure of simple transposon screens to find these immunoregulatory genes may be due to redundancy of TcpB/BtpA and BtpB function. The significant increase in Brucella survival in MyD88-/- knockout mice may also be due to the interaction between BtpA/BtpB and MyD88, as opposed to TLR4-/-/TLR2-/- knockout mice, in which no significant increase over wild-type was observed.68 The proinflammatory response that develops as a result of decreased ER stress and TLR-induced innate immune response may be negatively. TLR4 signaling during infection is restricted by the presence of elongated fatty acid chains that reduce the toxicity of the LPS (blue studs on Brucella surface) and by blocking downstream IKK phosphorylation via MyD88 binding (blue squares) with Brucella TIR-containing proteins, BtpA and BtpB (red squares), leading to enhanced polyubiquitination and degradation of MAL. However, the T4SS (VjbR-controlled expression) effector VceC (red oval) stimulates an innate immune response via interaction with BiP, an ER molecular chaperone (green squares) to release and phosphorylate IRE1 to promote mRNA splicing of XBP1 and activation of UPR. IRE1 phosphorylation also promotes the proinflammatory response via the release of NF-kB from the complex with IkBa (maroon crescent). The critical distinction between these two pathways may reside in the timing of activation. Inhibiting the host early or MyD88-mediated response may promote acquisition of a replicative niche, whereas the delayed T4SS-mediated VceC effector (VjbR-controlled expression) response may enhance the spread of the organism. Solid arrows indicate Brucella-mediated activation, whereas dotted arrows indicate Brucella-mediated inhibition. ER, endoplasmic reticulum; IkBa, inhibitor of kB protein a; Ikk, IkB kinase; IRE1, inositol- requiring enzyme 1; LPS, lipopolysaccharide; MAL, MyD88-adaptor like; MYd88, myeloid differentiation response gene 88; T4SS, type IV secretion system; TIRAP, Toll-IL-1 receptor domain-containing adaptor protein; TLR4, Toll-like receptor 4; TNF-a, tumor necrosis factor a; UPR, unfolded protein response; XBP1, X-box binding protein1,32
Development of protective Immunity against Stealthy Brucella
Mice, guinea pigs, ruminants, nonhuman primates, and humans are just a few of the diverse animal models used to study the prevention of Brucellosis. Numerous investigations, particularly those focusing on the functions of CD4 and CD8 T cells, have demonstrated the significance of a T helper cell type 1 (Th1) response against Brucella, even though these findings occasionally conflicted. Although natural killer (NK) cells have been demonstrated to be crucial in various hosts, the mouse model has little need for them.71 However, the effectiveness of the T helper cell type 2 (Th2) humoral immune response is still unknown,77 and the efficacy of rough Brucella vaccines contradicts the role of anti-LPS antibodies in protective immunity. Passive transfer experiments have suggested that antibodies against LPS (O- polysaccharide) may contribute to protection. Cytokines, which mediate both innate and adaptive immune responses, are the main players in the defence against Brucella. A Th1 response and the generation of interferon-g, which activates macrophages, are brought on by the IL-12 that B cells and macrophages release. Macrophages and NK cells create tumour necrosis factor-a, which increases the activity of interferon-g. According to certain research, IL-1 dependent colony-stimulating factor induction results in an increase in neutrophil and macrophage infiltration into the spleen.78 This event might also provide light on the function of T cell-produced IL-6. It is discovered that infected hosts' splenocytes had higher levels of the mRNA for IL-2, interferon-g, and IL-10 and lower levels of IL-4, which is consistent with a Th1 response. Due to the suppression of a protective Th1 response, a high level of IL-10 that was discovered later in the infection may assist Brucella's ability to evade immune surveillance.81 It's intriguing how different vulnerable hosts' cellular and humoral immune responses are to similar Brucella strains. The identification of trustworthy correlates of immune protection in manipulable model animal systems has been made difficult by this perplexing element of Brucella immunobiology. In most cases, it was assumed that Brucella had intrinsic resistance to other elements of the innate immune system, such as complement, opsonins, phagocytic cells, innate lymphocytes, cytokines, and other barriers, which give passive resistance to intracellular killing processes. It is becoming more evident that resistance mechanisms alone are insufficient for the success of infection, however, given the significance of the T4SS to the long-term success of infection. The immediate goal of Brucellae and other intracellular pathogens is to create a replicative niche and long-term persistence. To this end, they alter the innate immune response. The organism sneaks inside host cells to dodge the innate immune response before limiting long-term protective immunity. From there, the organism regulates bacterial reproduction, intracellular trafficking, and elements of protein secretion,31,40 which eventually changes how the innate and adaptive immune responses develop.82 The failure of the adaptive immune response, which is partly regulated by the suppressed innate immune response, to provide long-term protection against Brucella infection.72,83–85 Through the interaction of TLR ligands, Brucella enters the host cell stealthily and without obvious activation of the innate immune response. There aren't many noticeable differences in the ability to regulate infection in knockout mice and animals lacking one or both of TLR2 and TLR4. In contrast, bacteria are more likely to infect cells lacking MyD88 by a factor of two.86 A redundancy in host functions may be the best explanation for this finding. It might also mean that the main objective is to prevent long-term adaptive immune responses rather than to provide aid in the early stages of infection. Evasion of the innate immune response triggered by the host may allow the organism to establish a foothold, whereas activation at a later stage promotes infection transmission. At least three variables, TcpB/BtpA, BtpB, and VceC, were shown to influence the innate immune response. Although several more effectors have been identified, their significance to the pathogen's survival has yet to be proven.23,25,31,40 However, it becomes obvious that the wild-type Brucella has a full complement of effectors at its disposal, and that any delay in the innate immune response brought on by these proteins may be managed to increase the possibility of more effective and secure vaccinations.
Therapeutic approaches based on Brucella pathogenesis
Combination antibiotic therapy is commonly used to treat brucellosis in humans. However, a therapeutic recovery from brucellosis is neither certain nor quick. This is due to the intracellular position, which restricts antibiotics' effectiveness. The course of treatment could be drawn out and come with unfavorable side effects. Relapse following an antibiotic course also poses a serious risk to the patient receiving treatment. For all of these reasons, a combination of medications taken over the course of at least 30 days is typically the recommended course of treatment.87 Each of these medications falls into the Food and Drug Administration pregnancy categories C (rifampin) or D (tetracycline/doxycycline), which are poor to unsuitable for use in pregnant women. New treatment ways to treat brucellosis are required to solve this issue. This goal has been made easier to pursue by recent developments in our knowledge of the molecular processes that govern interactions between Brucella and host cells. For instance, Baron et al.88 have led the discovery of brand-new antivirulence substances that focus on virulence functions while protecting vital cell processes. The antivirulence strategy has the benefit of preventing the development of antibiotic resistance because compounds that do not target critical processes will not have this effect. A high throughput small molecule screen was conducted for compounds that inhibited the function of VirB8,89 a crucial part of the bacterial T4SS that is crucial for Brucella virulence and pathogenicity,90 as well as a variety of other animal and plant pathogens, in order to find novel compounds that reduce Brucella virulence. This project found numerous effective and targeted inhibitors of T4SS activity. The mechanism of action of this prospective treatment was disclosed by X-ray crystallography and docking investigations, which showed that VirB8 inhibitors attach to a surface groove opposite the dimerization interface.89,91
Vaccine approaches based on brucellosis pathogenesis
Heat-killed Brucella, crude extracts, subunit, and DNA vaccines are examples of historically non-viable Brucella vaccines with low protection rates. This low level of protection is mostly attributed to the live, attenuated vaccines’ inferior ability to elicit an efficient Th1 response (LAVs).92 In contrast, LAVs have a long history of effective usage against brucellosis and other intracellular infections in target species, such as ruminants, as well as in laboratory animal models. But because it poses a risk of relapse into severe virulence or persistent infection when administered to humans, care must be taken when developing LAV. It has been discovered that controlling animal brucellosis offers significant protection against human brucellosis. The two strains are distinguished from RB51, a rough strain that lacks the O-polysaccharide, by their smoothness (expression of intact LPS with O-polysaccharide). As a result, S19 and Rev.1 vaccinations offer greater protection but also exhibit high levels of human pathogenicity and are therefore ineffective against gravid female animals. RB51 is thought to be safe for use in pregnant women and does not cause the O-polysaccharide antibodies needed to discriminate between animals that have received the vaccine and those that have been exposed to the field strain. Although these vaccinations have distinct immunological potential differences from one another, no marker or correlate that can be utilized to forecast immune protection has been found. Simple point mutations or genomic rearrangements, such as gene deletion, can result in reduced pathogenicity. Even though a mutant with a full gene deletion already has a low chance of reversion, the chance of reversion to virulence can be made infinitesimally small by adding a secondary mutation. In an ideal scenario, the new mutation would disrupt the same pathway as the primary mutation, acting merely as a backup to prevent reversion and having no adverse effects on protective immunity or further virulence decrease. It goes without saying that caution must be used to strike a balance that supports life enough to improve immune protection without posing a risk of causing disease. Encapsulation of the attenuated vaccination strain to release the organism over time and to provide the added benefit of delivering a natural booster response is one method for accomplishing this objective.69,99 Using highly attenuated LAVs, this method dramatically improves immune protection by using a vaccine depot from which the attenuated Brucella is gradually released over a 30-day period.100 Additionally, the dependence of Brucellosis on the UPR, namely IRE1a, has been brought about by recent discoveries in cell biology.20,43 In an endeavour to develop LAVs that provide increased immune protection, this reliance may be abused. The proinflammatory response is synergistically stimulated by ER stress and TLR signalling.101 Recognizing vaccination candidates that do not limit the innate immune response and, as a result, create an effective adaptive immune response without raising safety or reversion issues is crucial for optimising the LAV vaccine approach.
Humans are a potential accidental host for Brucella species infections. Although there are 500,000 new human infections each year, there is no patient-friendly medication or vaccine for humans. A direct risk to public health is also posed by the threat of deliberate release, which cannot be reduced by the customary method of vaccinating animals to protect people. Pyrexia, diaphoresis, tiredness, anorexia, myalgia, and arthralgia are examples of non-specific influenza-like symptoms seen in people. Because Brucella can survive inside of the host cells, chronic infection is possible. To eventually induce cardiovascular, hepatic, lymphoreticular, neurological, and osteoarticular illnesses, Brucella are dispersed through the lymphoreticular system. Mucosal macrophages and DCs endocytose Brucellae as they swiftly move over the mucosal epithelial layer. Once Brucella has become accustomed to living inside macrophages, it can continue to live inside cells indefinitely. However, it can also spread throughout the body to infect other preferred target cells or tissues, including the foetal lung, male genitalia, skeletal tissues, endothelium, reticuloendothelial system, and placental trophoblasts. Only recently have precise processes for blocking intracellular trafficking to the lysosome, interacting partners' mechanistic steps, enzymatic activities, and protein changes been documented. It was discovered that subversion of the host UPR proteins was essential to Brucella's intracellular existence.102
According to recent research, Brucella manipulates host IRE1a signalling pathways to establish an intracellular niche that facilitates food uptake, pathogen multiplication, or pathogen cell-to-cell dissemination. It is possible to improve the design of brucellosis vaccines, improve biomarker diagnostics, and find particular druggable targets while minimising the issue of antibiotic resistance by carefully combining holistic systems biology with conventional reductionist techniques. Comprehensive knowledge of host and pathogen genes and pathways has been produced by current reductionist methodologies, but a greater understanding of how these genes interact at the level of the entire host system is still lacking. There is currently no sign or correlate that can be used to predict immune protection in animals, despite the fact that there are clear differences in immunological potential among the available vaccines. In target species as well as in experimental animal models, LAVs have a long history of efficacy against brucellosis and other intracellular infections. Finding vaccine candidates that don't limit the innate immune response and, as a result, elicit an efficient adaptive immunological response without raising safety or reversion issues is essential for an ideal LAV development strategy for humans. In order to produce brucellosis vaccines and treatments that are safer and more protective, Brucella possesses a full complement of effector proteins that inhibit the innate immune response.
We can therefore draw the conclusion that despite more than a century of research, human and animal Brucellosis continues to pose a serious threat to human health and the economics of life. Furthermore, there are currently no patient-friendly treatments or safe, effective vaccinations for human brucellosis. Additionally, low-cost bedside differential diagnosis is required for brucellosis. However, opportunities for identifying novel therapeutic targets and performing reverse vaccinology and other postgenomic tools to develop secure and efficient subunit or human LAVs are significantly increased with the emergence of clearer understandings of the pathology and molecular pathogenesis of brucellosis.
None.
The authors declare that there are no conflicts of interest.
None.

©2022 Ahmed. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.