eISSN: 2373-6372


Case Report Volume 14 Issue 6
1Unidad de Gastroenterología y Endoscopia Digestiva, Hospital Universitario San Ignacio, Bogotá, Colombia
2Departamento de Medicina Interna, Pontificia Universidad Javeriana, Bogotá, Colombia
3Hospital Universitario San Ignacio, Bogotá, Colombia
4Departamento de Cirugía Oncológica, Pontificia Universidad Javeriana, Bogotá, Colombia
Correspondence: Rafael Gregorio Peña Amaya, Cra 7 # 40-62. San Ignacio Hospital, 6th floor. Bogotá, Colombia
Received: September 26, 2023 | Published: December 29, 2023
Citation: Amaya RP,Vargas RD, Ladino AC, et al. Esophagogastric anastomotic leakage and anastomotic fistula: what can we do when endoluminal vacuum therapy fails? Gastroenterol Hepatol Open Access. 2023;14(6):197-201. DOI: 10.15406/ghoa.2023.14.00567
Anastomotic leak and anastomotic fistula after esophagectomy are frequent and detrimental complications after esophagectomy. Endoluminal vacuum therapy has been used as alternative treatment for these type of complications refractory to use of lumen-apposing metal stents and other endoscopic modalities. In this report, we describe successful endoscopic closure of anastomotic leak and anastomotic fistula with fibrin sealant as adjuvant therapy after vacuum therapy failed to completely treat these complications.
Keywords: anastomotic leak, anastomotic fistula, endoscopy, fibrin sealant, vacuum therapy
Malignant tumors of the esophagus remain the seventh most common cancer worldwide, with a reported global incidence of 604,100 new cases every year, while also being the sixth one with a higher mortality rate.1 Definite curative treatment can be achieved with different strategies, surgery being one of them. Esophagectomy is a technically difficult surgery associated with a high rate of complications,2 one of the most important being that of anastomotic fistula, which has a reported incidence between 1% and 30%.3 Anastomotic leakage is defined as full-thickness gastrointestinal defect involving the esophagus, anastomosis, staple line or conduit, irrespective of presentation or method of identification,2 with a reported incidence between 11.4% and 21.2%.4 Management of this complications is not standardized, possibly because of its vast range of presentation and diverse clinical spectrum. However, it is established that symptomatic leakage or leakage associated with complications should be actively treated through endoscopic management or surgery.5 We present a case of esophagogastric anastomotic leakage and anastomotic fistula that could not be fully treated with endoluminal vacuum therapy (EVAC) and adjuvant therapy with fibrin sealant (TISSEEL®) was required.
A 68-year-old man with a history of gastroesophageal junction carcinoma underwent neoadjuvant chemotherapy and subsequent radical esophagectomy. Post-surgery, the patient developed empyema, and an esophagogastric anastomotic leakage was suspected. Revision surgery revealed no clear leakage but pleurectomy and decortication were performed. Esophagogastroduodenoscopy confirmed an anastomotic leakage covering 40% of the anastomotic end (Figure 1), extending into the mediastinum. Another endoscopy identified leakage compromising approximately 50% of the anastomotic end. A 15 cm lumen-apposing metal stent (LAMS) was inserted, and the patient received antibiotics, enteral nutrition, and was placed on nil per os (NPO) status. Two months later, the LAMS was removed, but two persistent leakage sites were found (Figure 2a). A new LAMS was inserted along with the ENDOVAC system using GranuFoamTM (Figure 2b). The E-VAC system was changed every 3-5 days for a month, totaling 7 replacements. During one replacement, upper gastrointestinal bleeding occurred and was managed with hemoclips. The patient underwent endoscopy again, revealing a persistent 3 mm leak. A new E-VAC system was positioned and replaced twice. Subsequent endoscopy showed no leakage, and the patient continued with the previous LAMS and enteral nutrition through jejunostomy.
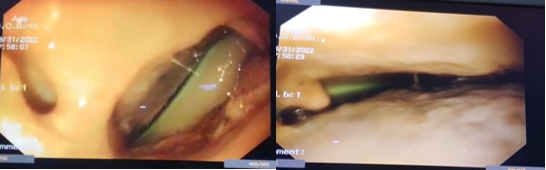
Figure 1 Anastomotic leak into the mediastinum where cavity drain tube could be observed. Endoscopic view.
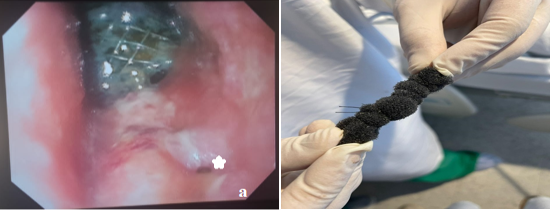
Figure 2a LAMS positioned with adjacent granulation tissue, *shows persistent anastomotic leakage. 2b GranuFOAMTM manually prepared for insertion with E-VAC system.
After comprehensive rehabilitation, a methylene blue test identified leakage below the LAMS. A new 12 cm LAMS was inserted, covering the leak and the patient remained on NPO status with enteral nutrition. After 28 days, the LAMS were removed, and no leakage was detected. An esophagogram confirmed an anastomotic fistula (Figure 3), and a new defect measuring 10 mm was managed with a new E-VAC system. The E-VAC system required 9 replacements, but the fistula persisted at a reduced size of 7 mm. Fibrin sealant was used for management. The patient resumed oral feeding after deglutition rehabilitation and showed improved dysphagia. During the last endoscopic follow-up, no leaks or fistulas were observed, indicating a successful procedure (Figure 4).
In this paper, we describe a case of anastomotic leakage and anastomotic fistula closure in a patient diagnosed with esophageal adenocarcinoma who underwent Ivor Lewis esophagectomy. Initially, the anastomotic leak was managed with FSEMS. However, it became evident that this strategy alone would not be sufficient to completely close the anastomotic defect. Therefore, EVAC therapy was implemented in conjunction with FSEMS. During regular in-hospital follow-ups, the anastomotic leakage showed gradual improvement, but unfortunately, an anastomotic fistula developed. EVAC therapy alone did not achieve complete sealing of the fistula, fibrin sealant was applied at the site of the remaining anastomotic fistula.
Anastomotic leakage has a mortality rate between 7.2% and 35%,6 while anastomotic fistula has a mortality rate between 0.8% and 11.6%.7 Management of these complications requires a multi-strategic approach and often involves a multidisciplinary team of physicians. The diverse presentation of anastomotic leaks and anastomotic fistulas, coupled with limited understanding of definitive risk factors and the absence of predictive models, poses a challenge when selecting between different management strategies. As a result, there is currently no standardized guideline for their treatment. Treatment strategy is typically based on factors such as the type and severity of the leakage, its size, underlying cause, timing of diagnosis, presence of symptoms, and the patient's nutritional status.
Price et al suggests classification of anastomotic leak in grade I (radiological), grade II (clinical minor), grade III (clinical major) and grade IV (conduit necrosis).8 Management should consist of observation (grade I), nil per os, oral antibiotics, wound drainage or CT-guided drain placement (grade II), esophageal stent placement, surgical debridement or anastomotic revision (grade III) and conduit resection with esophageal diversion (grade IV). Endoscopic treatment is less morbid and safer than surgery.9
Endoscopic techniques include positioning of stents, clipping with over-the-scope-clip (OTSC), suturing with overstitch system, EVAC therapy, stent-over-sponge (SOS) therapy and the use of sealant. Stent therapy consists of positioning a metallic prosthesis in the esophageal lumen to cover the defect until tissue healing is complete.10 FSEMS have lower rate of tissue overgrowth but higher risk of migration.11 This can be overcome with endoscopic clipping of the stent12 or with stents of larger diameter.13 There has been conflicting evidence regarding the role of stenting in cases where the anastomotic leak exceeds 30% of the circumference,5 however, it is still accepted as a viable strategy when the leak is no greater than 70%.10,14 It is recommended that stent treatment be initiated within the first 24 hours of an anastomotic leak, as delays beyond this timeframe have been associated with a higher incidence of negative outcomes. There is evidence supporting the use of stent treatment for anastomotic leaks following Ivor Lewis esophagectomy, with favorable long-term outcomes.15 Median treatment time of stent has been reported between two and four weeks, and appropriate time of removal has been reported between two weeks for anastomotic leak and four weeks for perforation.16 There are factors associated with a higher risk of procedure failure, such as proximal cervical leakage and stent positioning close to the esophagogastric junction. These factors are linked to a higher incidence of pain, aspiration, and globus sensation, all of which can make the procedure intolerable for the patient.4
EVAC therapy consists of placing an open-pored polyurethane sponge via endoscopy into the esophageal lumen or intracavitary space. The sponge is connected to a nasogastric tube, which is then connected to a low vacuum drainage system. Continuous negative pressure is applied to suction and debride underlying tissue, facilitating constant secretion drainage, reducing bacterial growth, promoting tissue proliferation, and improving microcirculation.4,17 EVAC therapy has been recognized as effective for managing and repairing upper gastrointestinal defects, including esophageal anastomotic leaks. EVAC therapy has been reported to have lower complications rates compared to FSEMS18 and a shorter duration of stay in the intensive care unit.19 Reported success rates has been as high as 86-100%17 but this may be an overestimation of studies with small number of patients. In the largest prospective cohort study of EVAC use among patients with perforation and anastomotic leakage the median therapy length was of 22 days,20 which seems to be comparable to length of use of metallic stents.
EVAC sponge needs to be changed regularly for it to be effective. The median day for endoscopic changes varies among different studies between 3 to 5 days,21 however, there have been reports of changeover as late as 7 days without apparent adverse effects.22 The rate of negative pressure in the vacuum system tends to vary between studies, with pressure ranging from 50-125 mmHg23 up to 125-150 mmHg.4 EVAC therapy is generally accepted as a treatment for anastomotic leaks that are less than 5 cm in diameter and in the absence of cavitation that could be worsened or perpetuated by the negative pressure vacuum system. EVAC therapy is not without complications, bleeding is a common adverse effect associated with the placement and replacement of the EVAC system, as the manipulation of inflamed and friable tissue can lead to bleeding. There have been reports of two patients who experienced death due to massive hemorrhage during sponge change, caused by sponge erosion into a major cardiovascular structure.20 This complication occurred in less than 1% of patients.17 It is recommended that an exhaustive and careful examination is done before sponge placement to avoid lodging near vascular structures. Bleeding control strategies include hemoclips and argon plasma therapy. Schniewind et al. revealed that EVAC had a lower mortality rate (12%) compared to surgery (50%) and stent placement (83%), while maintaining a higher success rate.24 Different techniques of placement of the EVAC system exist, a conventional one22 and a recently described modified one.17 There are no studies comparing the different placement techniques, and thus, there is no information regarding differences in outcomes. Therefore, we recommend that endoscopists should use the technique they feel most confident and experienced with.
Fibrin sealants are products that stimulate the final stage of the coagulation cascade and are indicated for use as an adjunct to hemostasis in adults and pediatric patients aged over 1 month.25 TISSEEL is a two-component fibrin sealant made from fibrinogen from pooled human plasma containing a fibrinolysis inhibitor called synthetic aprotinin, and thrombin. Tissue adhesives have been used in gastrointestinal surgery to prevent or manage anastomotic leakage, as they contribute to wound healing by forming a tight junction of the anastomosis.25 Fibrin sealants have also been used in treatment of anastomotic fistula by closing the defect by acting as a clot and by promoting polymerization.26 They are naturally degraded by the fibrinolytic action of the body within an average time of two weeks. Multiple studies have addressed the benefits of using fibrin sealants in cases of anastomotic leakage,25,26 however, other studies have reported that the routine use of fibrin sealant in Ivor Lewis esophagectomy did not have impact on the reduction of post-operative anastomotic leak.27 Application of fibrin sealant to sutured anastomosis in lower colorectal surgery has resulted in a decrease in anastomotic leakage and a reduction in overall cost.28 Contraindication for the use of TISSEEL is hypersensitivity to its components, particularly apotrinin, which has been reported as an allergen. Despite rigorous screening methods for pooled plasma used in the synthesis of these agents, infections have been reported with the use of fibrin sealants.
Procedure description
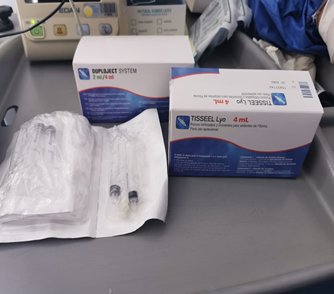
Figure 5 TISSEEL kit containing sealer protein, fibrinolysis inhibitor, thrombin and calcium chloride solution.

Figure 6 Sealer protein (a), fibrinolysis inhibitor (b), calcium chloride solution (c), thrombin (d).
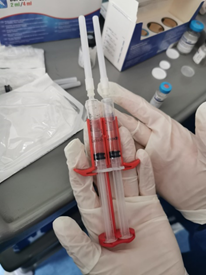
Figure 8 Syringes containing the sealer protein solution and the thrombin solution fastened in the Duploject applicator.
We employed a combination of FSEMS, EVAC therapy, and sealant therapy. Combination therapy of FSEMS and EVAC is known as the SOS technique, which has been reported for the management of complex leakages.29 SEMS ensures sponge adherence to the affected tissue, optimizing suction efficacy and direction. Remaining anastomotic fistula was subsequently managed with fibrin sealant. Long-term follow-up confirmed complete resolution of the anastomotic leakage without any complication. During the latest follow-up, patient reported eating and remained asymptomatic.
Currently, management of anastomotic leakage and anastomotic fistula involves implementation of various strategies that should be carefully employed by a multidisciplinary team of physicians. Management of complex leakages may require the use of multiple modalities, including FSEMS, EVAC therapy, and fibrin sealants. The combination of these modalities has shown to be safe and effective in the treatment of anastomotic leakages and anastomotic fistula following esophagectomy, with a low rate of complications.
None.
The authors declare no conflicts of interest.

©2023 Amaya, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.