eISSN: 2373-6372


Case Report Volume 4 Issue 3
1King Fahad Specialist Hospital, Saudi Arabia
2Qatif Central Hospital, Saudi Arabia
3AlZahra Hospital, Saudi Arabia
Correspondence: Ahmed H Al Salem, AlZahra Hospital, PO Box 61015, Qatif 31911, Saudi Arabia, Fax 96613860009
Received: December 22, 2015 | Published: March 11, 2016
Citation: ssa HAlwabari A, Alabdrabnabi F, Bseiso B, RiadAlnassralah et al. (2016) Endoscopic Ultrasound-Guided Pancreatic Pseudocyst Drainage in Children. Gastroenterol Hepatol Open Access 4(3): 00100. DOI: 10.15406/ghoa.2016.04.00100
Background: Endoscopic drainage of pancreatic pseudocysts is well established in adults and known to be associated with complications including perforation and hemorrhage. One reason for this is that endoscopic drainage is a blind procedure and to overcome this, EUS guided drainage was established. However, there are limited data regarding this procedure in the pediatric age group. The reasons for this are the limited number of cases seen in children and the lack of experienced pediatric gastroenterologist with this technique. Add to this another technical limitation with EUS is that the therapeutic echo endoscope is 14.6 mm in diameter and has a rigid tip that restricts its use in young children.
Patients, methods and results: This report describes a 4-year old child weighing 14 kg with a large pancreatic pseudocyst (8x8x12cm) that was treated successfully using adult endoscopic ultrasound scope (The Olympus EVIS LUCERA Ultrasound Gastrovideoscope GF-UCT 260, 14.6 mm distal tip).
Conclusion: Endoscopic drainage of symptomatic pancreatic pseudocysts can be achieved safely and effectively in children using the adult EUS scope.
Keywords: EUS, drainage, pancreatic pseudocyst, children, adult endoscope
Pancreatic pseudocyst is relatively rare in the pediatric age group and develop following acute or chronic pancreatitis, pancreatic trauma, or pancreatic duct obstruction.1,2 Currently, at least 3 major forms of therapy are available: percutaneous drainage, surgical intervention and endoscopic drainage.3–8 Controversy exists concerning which of these techniques should be offered to the patient as initial therapy. The recent advancement in endoscopic ultrasound (EUS) made it possible not only to diagnose but also to drain pancreatic pseudocyst.9,10 Drainage of pancreatic pseudocysts under EUS guidance is a well-established procedure for adults with pancreatic pseudocyst but there are only few reports using EUS drainage for pancreatic pseuodocyst in children and these are mostly case reports or small series.11–15 The main difficulty in using EUS guided drainage of pancreatic pseudocysts in children is the lack of experience due to the limits number of cases in children as well as the limited availability of EUS scope suitable for children and the technical limitations using the large size adult echoendoscope. This report describes a case of pancreatic pseudocyst in a child that was drained successfully using adult EUS guidance.
A 4-year-old boy weighing 14 kg, presented initially with abdominal pain, distension and vomiting of few days duration. Clinically, he looked sick, dehydrated, in respiratory distress, and his abdomen was markedly distended with severe tenderness all over the abdomen. The initial investigations in the local hospital showed WBC 4.6, Hb 12.5 g/dl, Amylase 2171, and lipase 1213. Abdominal ultrasound revealed pancreatic pseudocyst with moderate ascites. Abdominal CT-scan showed a large pancreatic pseudocyst (12X8X13cm) and massive ascites (Figure 1).
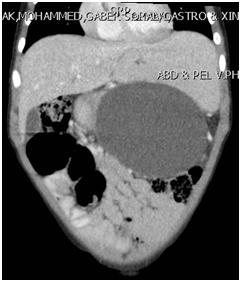
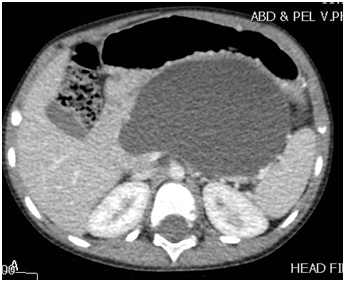
Figure 1A & 1B CT-scan showing a large pancreatic pseudocyst.
Note: The well formed wall and its adherence to the gastric wall.
US guided drainage was done for both the ascites and the pancreatic cyst. He was kept NPO, on TPN and given antibiotics and Octreotide. A follow-up CT scan showed persistent pseudocyst but smaller in size (4.3X2.6X1.8 cm) with minimal free fluid in the abdomen, so the drain was removed and he was started on low fat diet which he tolerated well. During follow up after 3 months, the patient was symptomatic complaining of abdominal pain and distension (Figure 2). A repeat abdominal ultrasound showed enlargement of the pancreatic pseudocyst size (5.9X4.6 cm) so he was referred to our hospital for evaluation and possible EUS guided transgastric pseudocyst drainage (Figure 3).
Upon presentation to our hospital, he was complaining of intermittent abdominal pain, post prandial abdominal fullness, however he was not ill and abdominal examination showed distended abdomen with fullness of the left upper quadrant and scars of previous drain insertion. His investigations showed WBC 11.86, Hb 12.8 g/dl, Amylase 123, and Lipase 818. CT Abdomen showed a significant increase in the size of the pancreatic pseudocyst (8x8x12cm). Consent was obtained from his parents for EUS guided cystogastrostomy. Prophylactic antibiotic using Ciprofluxacin was given before and after the procedure for three days. The Olympus EVIS LUCERA Ultrasound Gastrovideoscope GF-UCT 260, 14.6 mm distal tip was introduced easily through the esophagus to the stomach. This showed a large homogenous cyst measuring 8X9 cm, with a clear wall that was adherent to the gastric wall with no intervening blood vessels. This was punctured with 19 G needle and 30 ml of clear aspirate was sent for chemistry and cytology. A guide wire with two loops was formed in the cyst over which a needle knife was used to puncture the gastric wall and create an opening which was dilated to 4 mm. Two pig tail stents 7 Fr 4 cm were placed to drain the cyst. Post drainage, he was doing well, with no signs of complications, and resolution of abdominal pain and distension. He continued to receive antibiotics and was discharge after 2 days of the procedure in a satisfactory condition. His amylase and lipase levels went down to 109 & 631 respectively. The amylase level in the fluid was 7425 u/l, normal CEA level and cytology showed cystic fluid with no malignant cells. Six weeks later, MRI showed complete resolution of the collection and a repeat EUS confirmed resolution of pancreatic pseudocyst. The stent was removed and currently the patient is well and asymptomatic (Figure 4).
Pancreatitis and pancreatic pseudocysts are relatively rare conditions in the pediatric age group. The common causes of pancreatitis and pancreatic pseudocysts in children are trauma, gallstone pancreatitis, hereditary pancreatitis and hyperlipidemia, viral, bacterial or toxin-mediated, and idiopathic pancreatitis. Trauma continues to be the leading cause of acute pancreatitis and pancreatic pseudocyst in children accounting for up to 50% of cases, with a significant proportion requiring surgical intervention.2,6,7 Generally, the treatment of pancreatic pseudocysts is conservative and interventions are required only in symptomatic cysts, large cysts or complicated cysts. Generally, conservative treatment is advocated for all pancreatic pseudocysts that are small in size (<5cm in diameter) and surgical intervention is advocated for large cysts to avoid the risk of spontaneous rupture. Currently, there are three treatment modalities for pancreatic pseudocysts. These include percutaneous drainage, surgical intervention, and endoscopic drainage.3-8,16 Controversy still continues concerning which of these treatment modalities should be offered to the patient as initial therapy.
Surgery is the traditional treatment for pancreatic pseudocysts both in children and adults. It is a major surgery that is known to be associated with morbidity and mortality. Currently, surgical drainage of pancreatic pseudocysts should be reserved only for certain selected cases. Percutaneous drainage of pancreatic pseudocysts is a much simpler procedure than the open surgical drainage and known to be associated with less morbidity but it requires prolonged hospitalization and have a higher rate of recurrence once the catheter is removed. Percutaneous drainage of pancreatic pseudocysts can be achieved under CT or US guidance where a pigtail catheter is placed percutaneously into the pseudocyst. When the drainage output becomes minimal, the catheter is removed. This technique also has a high risk of infections. The catheter tends to block repeatedly and may require repositioning and exchange. Add to this the fact that the parents may not be able to manage the catheter at home and requires prolonged hospitalization and increased cost.8–10
The recent advancement in endoscopic techniques has made endoscopic drainage of pancreatic pseudocyst a better alternative than the open surgical approach.4–6 Endoscopic drainage has a high success rate and when compared to surgery it has a low complication and mortality rates. The creation of a fistula tract between the pancreatic pseudocyst and stomach (cysto-gastrostomy) and rarely between the pancreatic pseudocyst and duodenum (cysto-duodenostomy) allows continuous drainage of the pancreatic pseudocyst leading ultimately to complete resolution and disappearance of the cyst. This procedure is however a blind one and known to be associated with complications including perforation and hemorrhage. To decrease these risks, the endoscopic ultrasonography guided drainage of pancreatic pseudocysts was developed which allows drainage under direct sonographic visualization.
Endoscopic ultrasonography (EUS) guided drainage of pancreatic pseudocysts is a new that aims to decrease the risks associated with the usual endoscopic drainage. This technique combine endoscopy with real-time visualization of the drainage procedure using endosonography. Currently, EUS guided drainage of pancreatic pseudocyst is well established in adults and several authors have described its safety and effectiveness and was shown to be superior to conventional endoscopy for transmutable drainage of pancreatic pseudocyst and comparable with surgical cystogastrostomy.11–15 However, in children the number of cases and reports are limited. Jazrawi et al.,9 reported satisfactory outcomes with EUS-guided treatment for pancreatic pseudocysts in 10 children, 8 of them underwent EUS-guided puncture and stent placement.9 Also, the median age of children in that study was 11.8 years and the median size of pancreatic pseudocyst was 7.8 cm. De Angelis et al.,17 reported 12 children with pancreatic pseudocyst, 4 of them treated successfully using miniprobe EUS guided drainage.17 Ramesh et al.,10 reported single-step EUS-guided drainage of pancreatic pseudocyst in 7 children (mean age 8.4 years) but 2 of them underwent repeat EUS-guided drainage due to lack of adequate resolution of pancreatic pseudocyst.10 The findings of these reports demonstrate that single-step EUS-guided drainage is a minimally invasive, safe, and a highly effective technique for the management of symptomatic pancreatic pseudocysts in children. The procedure was technically feasible in children as young as 6 years and precluded the need for a surgical cystogastrostomy in all of the patients. The patients treated with EUS guided drainage of pancreatic pseudocysts had a significantly better quality of life, shorter length of hospital stay, and the technique was less costly when compared with the open surgical or laparoscopic drainage of pancreatic pseudocysts. In the pediatric age group, the lack of skilled pediatric gastroenterologists who are experienced with EUS and skilled to perform EUS guided drainage of pancreatic pseudocysts is a limiting factor. Another technical limitation with EUS is that the therapeutic echoendoscope is 14.6 mm in diameter and has a rigid tip with limited endoscopic room that restricts its use in extremely young children. Presently, there are no echoendoscopes designed specifically for use in very young children. Our patient is 4 years old and weigh only 14 kg. The adult endoscopic ultrasound scope size 13 mm was used and this proved to be feasible and safe not only to diagnose pancreatic pseudocyst but also to endoscopically treat it.
None.
Author declare that there is no conflict of interest.
None.

©2016 ssa, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.