eISSN: 2373-6372


Research Article Volume 13 Issue 5
University of Science Arts and Technology, USA
Correspondence: Orien L Tulp, University of Science Arts and Technology, Olveston, Montserrat, BWI. MSR1110, USA, Tel 664- 491-5364
Received: September 03, 2022 | Published: September 20, 2022
Citation: Tulp OL. Effect of delayed luminal carbodhydrate uptake via α-glucosidase inhibition on plasma glycemic parameters and lipid profiles in adult wistar fatty rats. Gastroenterol Hepatol Open Access. 2022;13(5):168‒173. DOI: 10.15406/ghoa.2022.13.00518
To determine the effects of delayed luminal glucose uptake on plasma glycemic and lipid profiles, groups of adult male lean and obese+NIDDM Wistar Fatty Rats (n=8 rats/group) were fed nutritionally complete diets where the carbohydrate was provided as cooked cornstarch (CS), sucrose (SU), or the SU diet containing an admixture of the luminal α-glucosidase inhibitor compound acarbose (ACB, 150mg/kg diet) from 22 to 30weeks of age. Measures of body weight, caloric intake, fasting Insulin to glucose (I: G) ratios, and plasma cholesterol (CHOL), triglyceride (TG) and Alpha (LDL) and Beta (HDL) Lipoprotein concentrations were determined after 8weeks of the dietary regimen. Food intake and weight gain of the obese+NIDDM phenotype was greater than the lean phenotype, increased with the SU diet. The weight gains of lean and obese+NIDDM rats fed the SU+ACB diet were similar to rats of the same phenotype fed the ST diet. Fasting I:G ratios were elevated in Obese+NIDDM and were similar to those of ST fed rats fed the ACB diet. Plasma CHOL and TG were greater on obese+NIDDM rats of both sexes, and ACB was associated with modest decreases in CHOL concentrations in both lean and obese+NIDDM rats. No adverse side effects of the ACB were observed in either phenotype. These results indicate that luminal inhibition of carbohydrate digestion via α-glucoside inhibition was associated with improvements in I:G ratios and in plasma lipid profiles that were similar to those observed with the complex CHO ST diet and may be a safe and useful adjunct in the treatment of NIDDM and other carbohydrate intolerant states.
Keywords: Obesity, NIDDM, Rats, CHO digestion, α-glucosidase, and LDL and HDL lipid profiles
NIDDM, non-insulin dependent diabetes; T2DM,Type-2 diabetes mellitus; CS, cooked cornstarch; DRTC, diabetes research training core laboratories; LDL, Low density
Type-2 diabetes mellitus (T2DM), commonly known as non-insulin dependent diabetes (NIDDM) is now one of the most prevalent metabolic diseases in the world The prevalence of obesity and its significant comorbidities are highly associated with NIDDM, in addition to multiple cardiovascular disorders and other pathophysiologic sequela.1,2 The incidence of these illnesses are now approaching severe proportions in Westernized Society with more than a third of the population considered overweight or obese, and has placed an enormous burden on the health care resources of those communities.3 In addition, the economic loss in the workplace places an additional strain on both health care resources and industrial productivity due to decreased individual and collective capacity when individuals are unable to attend to their workplace obligations.1,2 Advances in industrialization have brought with them substantial changes in diet preferences and nutritional practices along with improved workplace conditions which have inadvertently changed key contributors to maintaining energy balance in a society that overall now tends to be more sedentary than in the past in many occupations. Accordingly, it is important to explore novel approaches to combat the emerging trends in disordered energy balance and their contributions to pathophysiologic sequelae which may result from the disordered parameters of energy balance. No single strategy has yet been demonstrated to combat the emerging trends in the obesity+NIDDM dilemma, but numerous animal models of obesity have now been developed, including the Wistar Fatty Rat used in the current investigation to provide insight into environmental and pharmacologic strategies to address the issues.
The ingestion of high carbohydrate, high glycemic index diets are often contraindicated in Obesity+NIDDM, where they are commonly associated with elevations weight gain and adiposity, in association with increases in fasting plasma triglycerides, cholesterol including the LDL fractions, fasting and glucose stimulated insulin concentrations and other stigmata of obesity+NIDDM.3 Therapeutic considerations include adoption of improvements in life style, and diet planning that focuses on a complex carbohydrate, modest fat diet, with special attention to ensure adequacy in fiber and micronutrient intake, all of which is programmed to generate a lower glycemic index diet while controlling caloric intake to match projected energy requirements of the individual. The added incorporation of a starch blocker agent such as acarbose, miglitol or other natural inhibitors of starch digestion via competitive inhibition of a-glucosidase and sucrase that may be additive to the luminal effects of the complex carbohydrate diet regimen may also be recommended by the supervising clinician.4 In clinical practice, a modest dose of acarbose equivalent to 50 mg before each mean has been found to lower hemoglobin A1c and attenuate excess weight gain in NIDDM patients, most of whom may present with varying magnitudes of overweight or obesity conditions. Although the weight loss effects tend to be modest in the short term, in the longer-term peripheral insulin sensitive may be improved and thus lead to improvements in the metabolic profile of the individual. There are several a-glucosidase inhibitor drugs available in addition to a broad assortment of naturally occurring food components mostly from vegetarian sources that may also bring about similar effects on α-glucosidase inhibition.4
From a physiological and nutritional regulatory viewpoint, the intestinal α-glycosidases catalyze the hydrolysis of α1–4 glycosidic bonds of polysaccharides, oligosaccharides and glycoconjugates and thus play critical roles in various biological processes, including carbohydrate digestion.4,5 In particular, mammalian α-glucosidases are located in the mucosal brush border of the small intestine where they rapidly and efficiently catalyze the end step of digestion of starch and disaccharides and represent the rate-limiting step in luminal glucose absorption. Excess ingestion of carbohydrates including glucose, sucrose and fructose are often overabundant in the typical Western diet. Inhibitors of α-glycosidases including acarbose and others found in common foods can delay the breakdown of carbohydrates in the small intestine via competitive inhibition and thus bring about a dose related decrease in postprandial blood glucose excursions and decrease post prandial insulin requirements since the luminal uptake of the newly generated glucose occurs as rapidly as it can be generated. Thus, therapeutic measures that can attenuate the postprandial excursions in glycemic responses to diet have great therapeutic potential in strategies to control the progression of sequela that may occur in obesity, NIDDM and other glucose intolerant states. Numerous phenolic compounds found of foods and their secondary metabolites can contribute to the modulation of the postprandial excursions in glycemia by competitive inhibition of glucosidase enzymes, including alkaloids, flavonoids, phenols, and terpenoids. Among the most active of such compounds is acarbose, miglitol, voglibose, and 1-deoxynojirimycin (DNJ), all of which are now commercially available as anti-glucosidase drugs useful in the treatment of NIDDM. A unique advantage of such therapy is that luminal absorption of the pharmacologic inhibitors is limited, thereby escaping most hepatic or renal actions that may accompany pharmaceutic agents and other complex organic residues that undergo luminal absorption and hepatic metabolism.4
The glucosidase enzymes including α-glucosidase and sucrase are strategically located in the brush border of the gastrointestinal tract, extending into the lumen, where they can make easy contact with the luminal digesta and where the local environment is at an optimal pH to facilitate the enzymatic digestion of starches contained in the now denatured and partially digested material by the brush border activity. The gastrointestinal absorption of glucose and other monosaccharides occurs rapidly in the proximal small intestine, and results in the virtual complete uptake of monosaccharide residues following luminal digestion of both simple sugars including sucrose and complex carbohydrates including starches. The rate limiting enzymes of starch are α-glucosidase and sucrase, which are located throughout the intimal lumen of the small intestine, with the greatest enzymatic activity in the most proximal sections including the duodenum. Chronic or long-term high carbohydrate diets can induce an increased activity of the luminal glucosidases, further exaggerating the post-prandial glycemic excursions.
The Wistar fatty Rat strain used in this study is a genetic animal model of obesity and NIDDM that displays numerous characteristics of obesity and NIDDM which resemble the obese-NIDDM characteristics of humans and other similar rat strains.5–7 Moreover, the metabolic characteristics are similar in nature and magnitude to the same stigmata as they occur in humans afflicted with symptoms of obesity and NIDDM including impairments in thermogenesis and energy balance.6–9 The prevalence of NIDDM ranks among the most common consequences of obesity, is increasing in its prevalence in many countries and once diagnosed often requires intense patient observation, monitoring and treatment in an attempt to minimize to the extent possible chronic pathologies which may develop progressively over time if left untreated. The epigenetic locus or loci that result in the expression of NIDDM stigmata in this and other rodent strains and in humans are complex and remain unclear. Thus, the purpose of the present study was to further characterize the effect of the obesogenic -fa trait on parameters glycemic state and lipid profiles using methodology as performed in our laboratory for many years.9
Groups of adult male lean and obese Wistar Fatty Rats (n= 8 rats/group) were obtained from the Diabetes Research Training Core Laboratories (DRTC), University of Indiana at 20weeks of age, and placed in hanging steel cages by littermate pairs (1 lean +1 obese) with initial free access to Purina Chow #5012 for two weeks and house water during their adjustment period to decrease the potential effects of travel and related stressors on experimental outcomes. During the first week, environmental conditions were adjusted to a reverse light cycle (dark 0800-2000 hours) maintained at 20±1°C and 50% relative humidity thereafter. At 22weeks age the diet was switched to a semisynthetic diet recommended by Michaelis et al at the Carbohydrate Nutrition Research Laboratory of the USDA in Beltsville, Maryland, USA.10
The USDA compounded Michaelis diet consisted of (w/w) 54% CHO as sucrose (SU) or cooked cornstarch (ST), 16% mixed fats as equal parts lard, beef tallow, corn oil and coconut oil, 20% protein as equal parts casein and lactalbumin, 4% essential vitamins and minerals, and 5% cellulose, and were fed from 22 to 30weeks of age. A third group of each phenotype was offered the SU diet with an admixture of 150mg Acarbose (ACB) as recommended by the manufacturer for use in animals from 22 to 30weeks of age and designed to deliver 5 to 10mg of ACB/kg BW/day. While this dosage is consistent with the dosage ranges used in clinical studies, direct correlations between optimal dosages in human and animal studies are only speculative at best. Body weights of rats and the amount of food consumed per day were weighed weekly to the nearest gram on an Ohaus animal balance as outlined by Vedula et al.13 At 30 weeks of age rats were sacrificed by decapitation, truncal bloods collected for determination of fasting levels of serum glucose, insulin, total cholesterol, triglycerides, and the Alpha and Beta fractions of the cholesterol lipoproteins, corresponding with the Low density (LDL) and the High Density (HDL) lipoprotein fractions, respectively. Glucose was measured via a glucose oxidase method and insulin via solid phase radioimmunoassay.14 Serum total cholesterol and the Alpha (LDL) and Beta (HDL) lipoprotein fractions were determined enzymatically following separation of the lipoprotein fractions affinity chromatography with the procedure of Buculo and David.15 Data were analyzed via standard statistical procedures including ANOVA and Student’s T test. The Institutional Animal Care and Use Committee approved the study.
Body weights and net weight gain of rats are depicted in Figure 1 respectively and indicate that the obese+NIDDM rats were heavier than their lean littermates and gained more weight during the 8 weeks of the study. He inclusion of Acarbose in the SU diet resulted in less weight gain, with net weight gains in both phenotypes that were of a similar magnitude as control animals fed the ST diet. The obese+NIDDM phenotype consumed significantly more food per day than their lean littermates (Obese+NIDDM = 70±6g/d vs Lean 46±4g/day, Figure 2A), while food intake relative to body weight was greater in lean than obese and was not affected by the ACB regimen (Figure 2B). Rats of both phenotypes gained more weight than rats of the same phenotype when consuming the SU vs, the ST diet. The daily food intake, reported as grams of diet consumed per kilogram of body weight are depicted in Figure 2B, and indicate that when expressed relative to body weight, animals of both phenotypes consumed more food per day relative to their body weights when fed the ST than the SU diet, and that the ACB diet was without significant effect on relative daily food ingestion in either phenotype. In addition, while obese rats typically demonstrated frank hyperphagia, but when expressed per unit of body weight, lean rats tended to consume modestly greater food intake relative to their body weights daily than their obese+NIDDM littermates, and consumed more when fed the ST than the SU or ACB dietary regimens, consistent with a CHO type effect on appetite, and that the ACB additive did not appear to affect net parameters of overall appetite in either phenotype.

Figure 1 Effects of phenotype and acarbose on weight gain in Wistar fatty Rats. Data are mean ±1 SEM, N= 8 rats per group.

Figure 2A Effects of phenotype, diet and acarbose on food intake in Wistar Fatty Rats. Data are mean ±1 SEM, N= 8 rats per group expressed as grams food per rat as the average of a three-day duration.
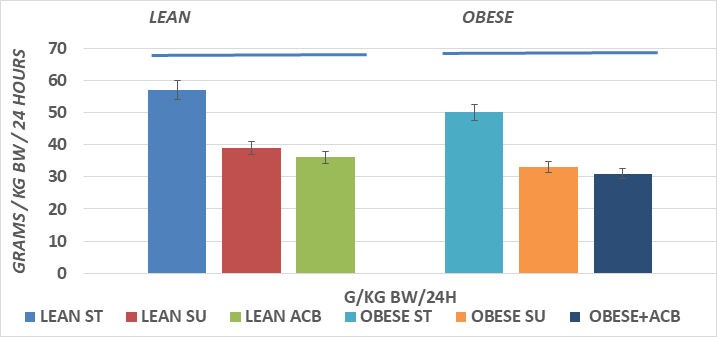
Figure 2B Effects of phenotype, diet and acarbose on food intake per kg of body weight in Wistar Fatty Rats. Data are mean ±1 SEM, N= 8 rats per group expressed as grams food per rat as the average of a three-day duration.
Serum total cholesterol concentrations are depicted in Figure 3 and indicate that total serum cholesterol was greater in the obese than the lean phenotype, and that the SU diet regimen resulted in a significantly greater serum cholesterol concentrations in the obese but not in the lean phenotype, where the serum cholesterol tended to trend only slightly greater in the lean SU group. The addition of ACB resulted in modestly lower serum cholesterol concentrations in both phenotypes but did not fully normalize the hypercholesterolemia in the obese+NIDDM phenotype. Serum Alpha and Beta lipoprotein concentrations are depicted in Figure 5A, and indicate that Alpha lipoprotein concentrations, corresponding to low density lipoprotein cholesterol (LDL) fractions, were greater in the obese+NIDDM phenotype than in their lean littermates, and increased further in both phenotypes in animals feed the SU diet. Addition of the ACB to the SU regimen resulted in a normalization of Alpha LP cholesterol in the lean phenotype but was without significant effect in reducing Alpha lipoprotein levels toward controls in the obese+NIDDM phenotype. Measures of the Beta Lipoprotein cholesterol, corresponding to High Density Lipoprotein cholesterol fractions were greater in the obese+NIDDM than the lean phenotype, and increased further when fed the SU diet in the obese+NIDDM phenotype. The ACB+SU diet resulted in modestly greater Beta-LP concentrations in lean and modestly lower Beta LP concentrations in the obese+NIDDM phenotype. The Alpha to Beta Lipoprotein ratios is depicted in Figure 5B and indicates the alpha to beta lipoprotein ratios were greater in lean than obese rats and were improved toward those of ST-fed rats when fed the ACB diet only in the obese phenotype.
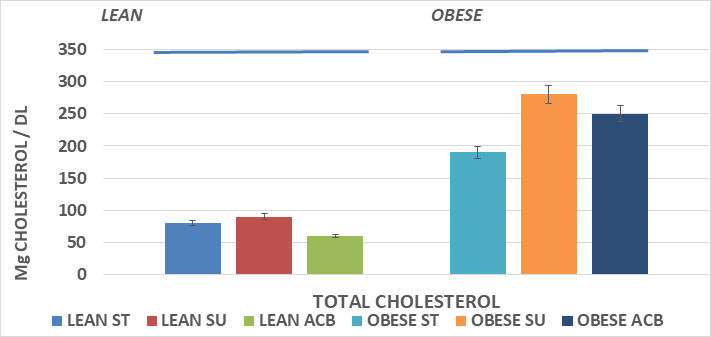
Figure 3 Effects of phenotype, diet and acarbose on fasting serum cholesterol in Wistar Fatty Rats. Data are mean ±1 SEM, N= 6-8 rats per group expressed as mg/dl.
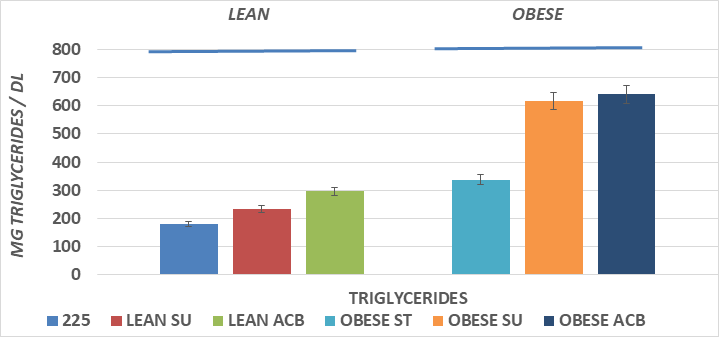
Figure 4 Effects of phenotype, diet and acarbose on fasting serum triglycerides in Wistar Fatty Rats. Data are mean ±1 SEM, N= 6-8 rats per group expressed mg triglyceride/dl.
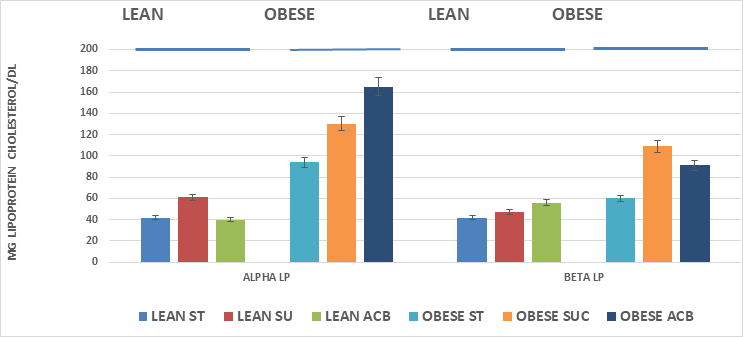
Figure 5A Effects of phenotype, diet and acarbose on fasting serum lipoprotein fractions in Wistar fatty Rats. Data are mean ±1 SEM, N= 6-8 rats per group expressed as mg/dl.
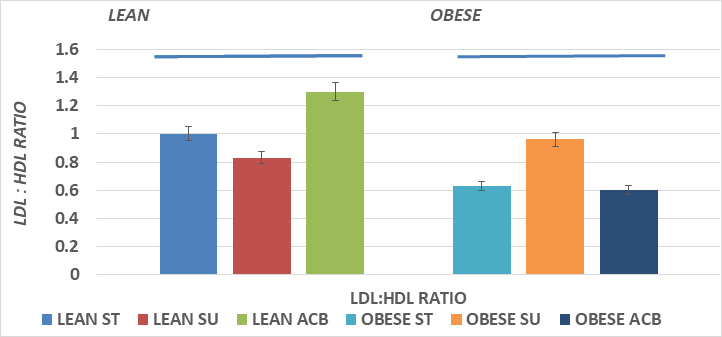
Figure 5B Effects of phenotype, diet and acarbose on fasting serum alpha to beta cholesterol ratio in Wistar Fatty Rats. Data are mean ±1 SEM, N= 6-8 rats per group expressed as mg lipoprotein fractions per dl.
Fasting serum triglyceride concentrations are depicted in Figure 4 and show that the total serum triglyceride concentrations of the obese+NIDDM rats were greater than their lean littermates, and that addition of the SU dietary regimen resulted in further increases n triglyceride concentrations in both phenotypes, with the greatest magnitude in the obese+NIDDM phenotype. The ACB SU diet was ineffective in lowering serum triglycerides in either phenotype.
The effects of phenotype and ACB on the fasting Insulin to Glucose ratios at the culmination of the study are depicted in Figure 6 and indicate that the I:G ratios of obese+NIDDM rats were greater than in the lean littermates and increased further when rats consumed the insulinogenic SU diet. The inclusion of ACB in the SU diet resulted in a return toward insulin to glucose ratios observed in ST fed animals of either phenotype but the modest dosage used in this study appeared to be insufficient to bring the insulin concentrations and the insulin to glucose ratios to within a normal range in the obese+NIDDM phenotype.
Acarbose is a complex oligosaccharide that acts as a competitive, reversible inhibitor of pancreatic alpha-amylase and membrane-bound intestinal α-glucoside hydrolase and has been found to be a useful agent in treating moderate severity NIDDM. In clinical trials lasting 12weeks or more, the HbA1c and glycemic responses typically demonstrated improvement over time while lipid profiles while improved, were less dramatic.18–29 The results of this study are consistent with previous clinical findings, and confirm that the 8-week trial of feeding a highly palatable high carbohydrate sucrose-laden diet to both lean and obese adult Wistar Fatty Rats with well-established NIDDM in the obese phenotype resulted in excess weight gain in both phenotypes, with the greatest gain by far among the obese+NIDDM phenotype. The SU diet also was associated with a predictable exaggeration of glycemic responses in the obese+NIDDM phenotype. The obese phenotype demonstrates significant insulin resistance, whether assessed by the fasting insulin to glucose ratio or application of the HOMA calculation, and which were only modestly improved when offered the acarbose regimen for only 8weeks. Both phenotypes developed predictable increases in fasting plasma triglyceride concentrations when fed the high sucrose regimen and the increases in the obese phenotype were considerably greater than occurred in the lean littermates. This observation is consistent with the greater insulin to glucose ratios, and the absolute hyperphagia (79g in obese+NIDDM vs 46g/day in lean phenotype) demonstrated in obese+NIDDM animals. These findings are consistent with those reported by Campion et al.16 who fed a nutritionally similar high carbohydrate regimen to a lean phenotype of male Wistar rats17 and reported a 1.5-fold increase in fasting triglyceride concentrations compared to the 1.3-fold increase in the lean phenotype of the present study. In the obese+NIDDM phenotype however, the SU diet resulted in a near doubling of plasma triglyceride levels compared to chow fed obese+NIDDM rats, and a 3.4-fold increase over levels observed in chow fed lean littermates. The differences in fold increase between the lean and the obese+NIDDM phenotype are likely due to the presence of the well-established metabolic stigmata present in the obese+NIDDM rats at the onset of the ACB treatment. Of interest, delayed luminal carbohydrate absorption via administration of a modest dietary dose level of acarbose a profound inhibitor of α-glucosidase and sucrase activity resulted in net weight gains that were similar to those of chow fed rats, but was without significant effect in lowering plasma triglyceride levels in either phenotype. Whether the failure of acarbose to effect changes in triglyceride levels following the high SU diet but did result in a normalization of weight gain might be secondary to metabolic effects resulting from pharmaceutically delayed carbohydrate digestion and subsequent luminal glucose absorption or if the responses may have been secondary to insulin lowering effects of plasma insulin following acarbose ingestion is unclear, but the effects noted are more likely due to the peripheral effects reflecting improved sensitivity and insulin actions in insulin-dependent peripheral tissues. In contrast, correction of the hyperlipidemia of Obesity+NIDDM once developed, likely takes a longer duration of treatment to return to pre-NIDDM levels in the presence of SU induced hyperphagia and insulin insensitivity with this mode of treatment. Vedula et al noted that the pattern of food intake differed when acarbose was included as an admixture thereby facilitating a comparable improvement in glycemic responses, and similar improvements in measured lipid parameters.13
The lipid profiles in the obese+NIDDM phenotype have been associated with increases in biochemical markers for free radical development and are clearly consistent with atherogenic lipid profiles, including elevations in both serum triglycerides and cholesterol, including the Low density (Alpha) lipoprotein fraction which have been associated with senescent alterations in the vascular intima.29–31 Although the lipid parameters were not remarkably improved in the obese+NIDDM rats following the ACB treatment, the excess weight gain of obese+NIDDM rats consuming the high sucrose diet was improved, suggesting relatively short duration of the therapeutic regimen may have been effective in attenuating the glycemic status, with some improvement in insulin sensitivity, but the normalization of the lipid profiles may require a longer treatment duration or a greater dose of the α-glucosidase inhibitor agent. In contrast, acarbose was effective in improving both glycemic and lipid parameters and in normalizing weight gain with the SU diet to that of ST fed controls. In contrast, acarbose treatment at the same level was effective in normalizing excess weight gain, serum triglycerides and the Insulin to glucose ratio in the lean littermates without demonstrable effects on food intake in either phenotype. Thus, the use of α-glucosidase inhibitors for the treatment of glucose intolerant conditions remains a useful approach in controlling the insulin dependent hyperglycemic sequelae of the obese+NIDDM phenotype of this strain and supports its usefulness in the treatment of NIDDM in humans. While individual adipose depots were not examined in this study, other studies in this strain noted marked increases in mass and cellularity in the visceral retroperitoneal depot, along with smaller increases in other subcutaneous depots when fed a similar diet.17,18 Thus, long term treatment with α-glucosidase inhibitors especially when combined with dietary intervention can bring about long term improvements in both glycemic and lipid profiles, and a likely decreased rate of progression of NIDDM and atherogenic markers of cardiovascular disease and senescence.22–31 Thus, the α-glucosidase competitive inhibitor Acarbose has been evaluated in several short to medium length clinical studies where it has been found to result in statistically significant improvements in glycemic parameters in NIDDM, including improvement in blood glucose and HbA1c levels when used as monotherapy in conjunction with dietary modification. Most studies have reported beneficial effects after 12 or more weeks administration of a dose of 50 to 100 mg before meals.22–29 In contrast, the effects of acarbose on lipid parameters have been variable, likely due to differences in patient populations, duration of pre-existing illness, dosages employed, and duration of treatment, and are likely attributable to an improved economy of insulin sensitivity and glucose utilization in peripheral tissues.
The short term administration of a modest dosage of the α-glucosidase inhibitor acarbose in the presence of a high glycemic index sucrose-enriched diet was found to be useful in attenuating the elevated insulin to glucose ratios, excess weight gain and increases in plasma cholesterol concentrations of the adult male lean Wistar Fatty Rats, but was only partially effective in correcting the same parameters in the obese+NIDDM phenotype where the most significant improvement was in sucrose-induced weight gain. In lean phenotype, the effects of the α-glucosidase inhibitor combined with the sucrose enriched diet resulted in improvements in excess weight gain and metabolic parameters that were similar to parameters observed in lean littermates fed the lower glycemic index starch diet for the same duration, consistent with typical dietary recommendations for consumption of complex carbohydrate diets for a variety of glucose-intolerant conditions. The same dietary and therapeutic regimen was only partially effective in the obese + NIDDM phenotype however, where the NIDDM and obese stigmata were already well established since adolescence and later adulthood at the start of the present study. Nonetheless, the strategy of delayed carbohydrate digestion via modulation of luminal α-glucosidase and sucrase activity as monotherapy to bring about an attenuation of the glycemic responses to consumption of simple carbohydrates including sucrose remains an attractive and useful pharmacotherapy mechanism to improve the metabolic and pathophysiologic stigmata of obesity, NIDDM, the premature progression of molecular changes associated with senescence, and in other glucose intolerant conditions in the obese and longstanding NIDDM of mild to moderate magnitude. Moreover, the clinical effectiveness of luminal glucosidase inhibitors on lipid parameters may be enhanced by the addition of a cholesterol lowering agent as has now been demonstrated in multiple clinical trials. The clinical efficacy of acarbose have now been demonstrated in numerous clinical trials and demonstrated effectiveness in treating obesity associated NIDDM.19–31
The author thanks Ms. Carole Stevens, Ms. Ollie Barbee, and Mr. Huang Peisong for assistance in data collection and animal husbandry, Dr S Dubin for veterinary support, and the late Dr O.E. Michaelis IV of the USDA Carbohydrate Nutrition Laboratory for the generous contribution of the semisynthetic diets used in this study.
Author declares there are no conflicts of interest towards the article.
None.

©2022 Tulp. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.