eISSN: 2373-6372


Research Article Volume 11 Issue 1
1Department of Hepatology and Gastroenterology, Egypt
2Department of blood bank, New General Hospital, Egypt
3Department of Radiology, Mansoura University Hospital, Egypt
Correspondence: Hany R Shabana, A/Professor of Hepatology & Gastroenterology, Internal Medicine Department,Specialized Medical Hospital, Faculty of Medicine, Mansoura University, Mansoura, Egypt, Tel +201060396985
Received: November 28, 2019 | Published: January 6, 2020
Citation: Shabana HR, El-Desoky AEE, Askar M, et al. Direct acting antiviral therapy of chronic hepatitis C may affect the progression of dysplastic nodules to hepatocellular carcinoma: A pilot study. Gastroenterol Hepatol Open Access. 2020;11(1):8-11. DOI: 10.15406/ghoa.2020.11.00406
In DAA drug therapy for chronic hepatitis C (CHC), one of the goals of treatment is to reduce the occurrence of liver cirrhosis related complications through viral clearance, the most important of which is hepatocellular carcinoma (HCC). HCC development in CHC cirrhotic patients may pass through two different pathways. One is the multistep carcinogenesis process, progressing from regeneration nodule (RN) to low grade dysplastic nodule (LGDN), high grade dysplastic nodule (HGDN) then HCC. Aim of the work: To assess the ability of DAA therapy of CHC patients to affect the progression of dysplastic nodules to HCC. Results: the DNs progressed to overt HCC in 5 of 12 cases who received DAA for CHC (38.4 %) during a median follow up period of 16 months (range from 8 to 33 months). Three of the progressed cases had achieved SVR12, while Two of them failed to achieve it. In the progressed cases, HCC was diagnosed at an early stage. Conclusion: DAA therapy of chronic hepatitis C patients with dysplastic nodules does not accelerate the progression of DNs to HCC. The progression of DNs to HCC is less than that reported for untreated patients. Also, the progression of DNs to HCC is not aggressive and all progressed cases are diagnosed at an early HCC stage. Further studies are needed to confirm these observations.
Keywords: DAA, HCC, Dysplastic nodules, HCV, direct acting antiviral, chronic hepatitis C
CHC, chronic hepatitis C; HCC, hepatocellular carcinoma; RN, regeneration nodule; LGDN, low grade dysplastic nodule; HGDN, high grade dysplastic nodule; DAA, direct acting antiviral; SVR, sustained virologic response; AASLD, American association for the study of liver diseases; MRI, magnetic resonance imaging; CT, computed tomography
In the era of interferon free direct acting antiviral (DAA) drug therapy for chronic hepatitis C(CHC), one of the goals of treatment is to reduce the occurrence of liver cirrhosis related complications through viral clearance, the most important of which is hepatocellular carcinoma (HCC). This goal was based on the effect of the older therapy of Pegylated Interferon and Ribavirin which resulted in reduction of HCC occurrence in compensated cirrhotic patients who achieved sustained virologic response (SVR). The rate of HCC occurrence was strongly decreased after SVR but not abolished with incidence of HCC ranging from 0.4 to 2%.1 Confirming this role for DAA needs longer time of follow up, especially for cirrhotic patients who have achieved SVR. Recently, some studies reported increased aggressiveness and rates of HCC recurrence in HCC patients who cleared HCV with DAA after achieving complete response to resection or local ablation of the tumor.2 Others concluded that cirrhotic patients treated by DAA are not at increased risk of developing (de novo) HCC compared to untreated patients.3 HCC development in CHC cirrhotic patients may pass through two different pathways.4,5 One is the multistep carcinogenesis process,5,6 progressing from regeneration or cirrhotic nodule (RN) to low grade dysplastic nodule (LGDN), high grade dysplastic nodule (HGDN) then HCC.4 The other pathways is the de novo carcinogenesis.
The prevalence of dysplastic nodules (DN) in patients with cirrhosis ranges from 14% (nodules ≥1cm) (7) to 37% (nodules ≥0.5cm).9 DNs are currently considered as pre-cancerous lesions.9–11 In general, DNs diameter range between 1 and 3 cm, with smaller lesions being consistent with RNs. DNs are defined as RNs containing atypical cells with nuclear crowding and architectural derangement and a variable number of unpaired arterioles or capillaries without definite histologic signs of malignancy.12 LGDN show normal hepatic architecture and vascular profile with a low malignant potential, slow and infrequent progression to HCC,13 whereas HGDN present some architectural distortion and more advanced atypia, with sinusoidal capillarization and an increased density of unpaired arteries, and are thought to progress to HCC more frequently than LGDN.14,15 Recently, the treatment of HGDN, such as surgical resection or ablative therapy, has been seriously considered.16 Masahiro Kobayashi et al. reported that HCC transformation rate from HGDN was 46.2% at 1 year, 61.5% at 2&3 years and 80.5% at 5years. The rate of HCC development from LGDN was 3.3% at 1year, 30.2% at 3 years and 36.6% at 5years. The rate of HCC development from RN was 2.6% at 1 year, 9.7% at 3 years and 12.4% at 5years. The authors concluded that HCC develops more often from HGDN than from LGDN and RN and that HGDN should be considered a precancerous lesion when it appears during follow up of chronic viral hepatitis or cirrhosis.17
To assess the ability of DAA therapy of CHC to affect the progression of dysplastic nodules to HCC. Also, the timing and pattern of HCC development in the progressed lesions will be assessed.
Thirteen CHC known genotype 4 patients (7 males and 6 females) with DNs discovered during their evaluation for DAA therapy were included in the present work. Twelve of them were treatment naïve and only one case was treatment experienced. CHC was diagnosed by the presence of Anti HCV Antibody using fourth generation ELISA and confirmed by RT PCR for HCV RNA. DNs was first discovered by routine real time (RT) abdominal ultra-sonography (US) and nature of the nodule was confirmed by contrast enhanced abdominal CT(CECT), dynamic contrast enhanced abdominal MRI or both of them , in highly suspicious lesions to exclude early HCC. Also, CECT and dynamic contrast enhanced MRI was done in CHC cases with baseline alpha fetoprotein (AFP) above 20ng/ml. The nodule was diagnosed as HGDN if it showed early contrast enhancement without washout on later phases, while it was diagnosed as low-grade nodule if it appeared hypointense.18 Hepatic functional reserve was evaluated before and after DAA therapy by calculating Child –Turcotte-Pugh (CTP) and MELD scores. The degree of liver fibrosis was evaluated before and after DAA therapy by calculating APRI, FIB4 and fibrosis index (FI) scores. AFP was evaluated before and after the end of DAA therapy. DAA therapy was prescribed according the available regimen at the time of patient presentation, being Sofosbuvir plus Ribavirin for 6 months at the start of the study, and then shifted to Sofosbuvir, Daclatasvir ± Ribavirin or Sofosbuvir, Ledipasvir ±Ribavirin for 3months when they became available in Egypt. In the last two regimens, when Ribavirin was not administered, treatment duration was extended to months.
The response to DAA therapy was evaluated by RT PCR at the end of DAA treatment & 12 weeks post treatment. SVR12 is defined as HCV RNA undetectable at or after 12 weeks post treatment.2 Adverse events including GI bleeding, marked ascites, hepatic encephalopathy and hepatorenal syndrome were recorded. Change of the nature and diameter of DN was evaluated by abdominal contrast enhanced CT and or MRI every 3months.1 The progression of DNs to overt HCC was diagnosed according to the practice guidelines of the American Association for the Study of Liver Diseases (AASLD) when arterial-phase enhancement and venous- or delayed-phase washout on dynamic computed tomography (CT) or magnetic resonance imaging (MRI) has occurred.19
The age of studied patients ranged from 46 to 70years (median age 69years, mean age 57years). The number of the DNs was single in 7 cases (53.8%) and multiple in 6 cases (46.2%).The DN diameter ranged from 1cm to 2.7cm with mean size of 1.43cm. As regards the radiological criteria, HGDNs were detected in 12 cases (92.3%), LGDNs were detected in one case (7.7%). Serum AFP ranged from 4.8 to 114ng/ml (mean AFP was 30.46ng/ml) and was more than 20ng/ml in 5 cases (38.7%). The CTP score ranged from 5 to 8 points. Ten patients (76.9%) belonged to CTP class A, while 3 patients (23.07%) belonged to CTP class B .The MELD score ranged from 0.37 to 12.19 points (mean MELD was 5.57). As regards the indirect serum marker of liver fibrosis, APRI ranged from 0.55 to 8.7 (mean APRI was 2.36), FIB4 ranged from 1.08 to 23.6 (mean FIB4 was 6.23) and FI ranged from 0.05 to 4.6 (mean FI was 2.92). DAAs were given for 6months in 5 patients (38.4%) and for 3months in 8 patients (61.6%).
Sofosbuvir 400mg, weight based Ribavirin daily for 6 months were given in 4 patients(31%), Sofosbuvir 400 mg, Daclatasvir 60mg daily± Ribavirin in 8 patients (61%), and Sofosbuvir, Ledipasvir combination plus Ribavirin daily for 3months in 1 patient (8%). The time interval from the diagnosis of the DN till the start of DAA ranged from 1week to 96 weeks (mean 17.7weeks). The follow up period ranged from 8 to 33months (mean 15.23months). At the end of DAA treatment, RT PCR was BDL in 13 cases representing 100 % of cases .SVR12 was achieved in 11 of 13 cases representing 84.6%. There was significant decrease of APRI score at the end of treatment (p=.05).There was significant decrease of both ALT and AST at the end of treatment (p=.006 and 0.035 respectively).There was significant decrease of AFP at the end of treatment (p=.016).As regards the serious adverse events during follow up, only bleeding from ruptured varices occurred in one patient (8%). As regards the change of the nature of the DN, it was changed to overt HCC in 5 cases (38.4 %), all of them occurred post treatment, and 3 of them had achieved SVR12, while 2 of them failed to achieve it (Figure 1). There was an increase of the DN diameter in 5 cases (38.4%), four of them (80%) had changed to overt HCC. There was a decrease of the DN diameter in 4 cases (30.7 %).There was disappearance of DNs in 4 cases (30.7%) (Figures 1 & 2).
DAA represent an evolution in management of CHC, with very high safety and efficacy profiles. Many of CHC patients who were PEG Interferon ineligible can now be treated by DAA. Despite the very high SVR rates achieved by DAA (more than 95%),20 the overall effect on liver cirrhosis related complications like development of HCC, is not well studied. Moreover, the treatment of CHC in patients with precancerous lesions like DNs, is not studied till now. These precancerous lesions are frequently encountered during evaluation of CHC patients for DAA treatment and the decision to start DAA or to wait is still an area of debate.
In the present work, we started the DAA treatment in CHC patients with DNs .The age of treated patient was older than 46 years, which is explained by the fact that CHC infection is a slowly progressive disease which needs decades to progress till appearance of DNs. The diameter of nodules ranged from 1 to 2.7cm, a diameter which distinguishes DNs from regeneration or cirrhotic nodules which are usually smaller than 1cm. The radiological criteria of the studied nodules were more similar to HCC than to RN or cirrhotic nodules. They showed arterial phase enhancement in 92, 3 % of cases, but, unlike HCC, there was no washout in the following phases of contrast enhanced CT and MRI, denoting HGDN nature of these nodules rather than LGDN. The overall SVR12 was 84.6% which is lower than expected because 31% of the cases were treated by Sofosbuvir plus Ribavirin for 6months (the first INF free regimen available in Egypt at the start of the study).However, this overall SVR12 was not so far from the results published by Esmat et al who reported SVR rates for treatment-naïve patients for 12=weeks using Sofosbuvir plus Ribavirin of 84%.21 Change of the nature of DN to overt HCC occurred in 38.4 % of cases (all occurred after the end of DAA treatment) during a median follow up period of 16months (range from 8 to 33 months). This figure is lower than that reported by Masahiro Kobayashi et al who reported that the HCC transformation rate of HGDN was 46.2% at one year in untreated chronic viral hepatitis patients with or without liver cirrhosis. This observation means that the rate of HCC transformation is lower in CHC patients with DN who were treated by DAA than untreated patients. This may be explained by the finding that DAA treatment resulted in significant improvement of hepatic inflammatory markers (ALT, AST), indirect serum markers of liver fibrosis (APRI) and AFP. It is worth mentioning that 100% of the cases who failed to achieve SVR12 had progressed to HCC, while only 30% of the cases who achieved SVR12 had progressed to HCC (Figure 1). This observation denotes that the progression of DN to HCC is associated with DDA treatment failure. Also, HCC developed in 50% of the cases who had advanced hepatic fibrosis or cirrhosis (F3-F4) at the start of DAA treatment, while it developed in 33% of cases who had lesser degree of hepatic fibrosis as estimated by FI (Figure 2). This observation means that the progression of DN to HCC was associated with more advanced liver disease at the start of DAA treatment. At the end of DAA, HCC developed in 75% of the cases who were CTP class B, while it developed in 25% of the cases who were CTP class. It also developed in 50% of cirrhotic patients; while it developed in 33% of patients with lesser degree of hepatic fibrosis as estimated by APRI .This observation means that the progression of DN to HCC was associated with more advanced liver disease at the end of DAA treatment. As regards the pattern of DN progression to HCC, 4 of the 5 cases (80%) who progressed to HCC showed increase of the nodule diameter, however, none of the progressed lesion exceeded 3cm i.e. small HCC. There was no portal vein thrombosis, lymph node or distant metastasis in any progressed case. This observation denotes that the progression of DN to HCC in CHC patients who were treated by DAA was not aggressive. Limitations of the present work were the limited number of patients, short follow up period, lack of pathological diagnosis of the nature of the focal hepatic lesion, lack of pathological diagnosis of liver fibrosis and cirrhosis.
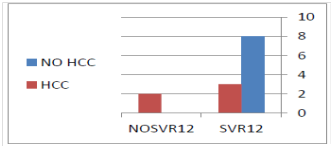
Figure 1 100% of the cases who failed to achieve SVR12 had progressed to HCC, while only 30% of the cases who achieved SVR12 had progressed to HCC.
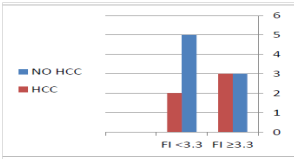
Figure 2 HCC developed in 50% of the cases who had advanced hepatic fibrosis or cirrhosis (F3-F4) at the start of DAA treatment, while it developed in 33% of cases who had lesser degree of hepatic fibrosis as estimated by FI.
Variable |
Result |
Age in years (range) |
46-70 |
Sex (male/female) |
7/6 |
Treatment (naieve/experienced) |
12/1 |
CTP class (A,B,C) |
10/3/0 |
Focal hepatic lesion |
7/6 |
Serum AFP(ng/ml)(range) |
4.8-114 |
MELD score (range) |
3.7-12.19 |
APRI (range) |
0.55-8.7 |
FIB4 (range) |
1.08 -23.6 |
FI (range) |
0.05 - 4.6 |
Antiviral treatment duration (6/3 months) Antiviral treatment type (SOF+Rib/SOF+DAC/SOF+LED+Rib) |
5/8
4/8/1
|
SVR 12W |
11/13(84.6%) |
DNs Progression to HCC |
5/13(38.4 %) |
Table 1 baseline criteria of the studied patients and their outcome
CTP, Child-Turcotte-Pugh; HGDN, high grade dysplastic nodule; LGDN, low grade dysplastic nodule; RN, regeneration nodule; AFP, alpha foetoprotein; MELD, Model of end stage liver disease; APRI, AST platelet ratio index; FIB4, fibrosis 4; FI, fibrosis index; SOF, sofusbuvir; Rib, ribavirin; DAC, Daclatasvir; LED, ledipasvir; SVR, sustained virologic response; DNs, dysplastic nodules; HCC, hepatocellular carcinoma
DAA treatment of chronic hepatitis C patients with dysplastic nodules does not accelerate the dysplastic nodules progression to HCC. The progression of dysplastic nodules to HCC is less than reported for untreated patients. Also, the progression of dysplastic nodules to HCC is not aggressive and all progressed cases are diagnosed at an early HCC stage. Further studies are needed to confirm these observations.
I thank my family and my colleagues in Hepatology & Gastroenteology unit of Internal Medicine department in Mansoura University, for their continuous support.
No conflicts of interests to declare.
None.

©2020 Shabana, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.