eISSN: 2373-6372


Research Article Volume 4 Issue 1
1Pharmerit International, USA (formerly of Adelphi Values, USA)
2Astellas Pharma Global Development, Inc., USA
3The Brod Group, USA (formerly of Adelphi Values, USA)
4Adelphi Values, USA
5Beth Israel Deaconess Medical Center, USA
6Astellas Pharma US Inc., USA
Correspondence: Bernhardt Zeiher, Astellas Pharma US Inc, 1 Astellas Way, Northbrook, IL 60062, USA, Tel (224) 205-8637, Fax (224) 205-8228
Received: December 14, 2015 | Published: January 28, 2016
Citation: Lasch K, Delgado-Herrera L, Waldman LT, Rosa K, Spears G, et al. (2016) Development of a New Instrument to Assess Stool Form and Consistencyin Irritable Bowel Syndrome with Diarrhea. Gastroenterol Hepatol Open Access 4(1): 00084. DOI: 10.15406/ghoa.2016.04.00084
Background: Available stool form scales do not optimally capture the continuum of stool consistency experienced by patients with irritable bowel syndrome with diarrhea (IBS-D). This paper describes the development of a new measure to assess stool form and consistency for IBS-D.
Methods: Descriptors for a new scale were selected by comparing spontaneous descriptions of stools from patients with IBS-D (Rome III) with spontaneous patient descriptions of images in the Bristol Stool Form Scale (BSFS) and an adapted BSFS. New images were prepared by an artist based on the final descriptors and literature images. The new Astellas Stool Form Scale (ASFS) was assessed in cognitive interviews.
Results: Spontaneous reports of stool descriptors from 50 patients with IBS-D were broadly grouped based on conceptual equivalence. From these, 8 final stool descriptors were selected, ranging from “like marbles or hard rocks” to “just liquid,” and an image was created for each descriptor. In 20 cognitive interviews, 75% of patients with IBS-D indicated that descriptors matched the appropriate images, 95% of patients indicated that there were enough images to depict each stool type, and 70% of patients indicated that all images were clear.
Conclusion: The ASFS, developed using spontaneous patient reports and rigorous qualitative methods, was well understood by patients with IBS-D and was relevant to their experience of stool form and consistency with IBS-D. Further evaluation is ongoing to confirm its utility in clinical research and in the clinical setting as a tool to monitor change in patients with IBS-D and to assess treatments.
Keywords: Irritable bowel syndrome; Irritable bowel syndrome with diarrhea; Patient-reported outcome; Stool consistency; Stool form scale
ASFS: Astellas Stool form Scale; BSFS: Bristol Stool Form Scale; CDC: Centers for Disease Control and Prevention; FDA: Food and Drug Administration; IBS: Irritable Bowel Syndrome; IBS-C: IBS with Constipation; IBS-D: IBS with Diarrhea; IBS-M: IBS with mixed stool pattern; IBS-U: unspecified IBS; PRO: Patient-reported outcome
Diagnosis and assessment of irritable bowel syndrome (IBS) are performed using symptom-based criteria such as the Rome III diagnostic criteria for IBS.1,2 Thus, well-defined and reliable patient-reported outcome (PRO) measures are essential for evaluating interventions for treatment benefit and monitoring disease status. IBS can be subtyped according to the predominant stool pattern: IBS with diarrhea (IBS-D), IBS with constipation (IBS-C), IBS with mixed stool pattern (IBS-M), and unspecified IBS (IBS-U; insufficient abnormality of stool consistency to meet criteria for the other 3 subtypes).2 However, more than 75% of patients will change subtype over a 1-year period.3 Since unstructured or informal patient descriptions of constipation and diarrhea may be misleading, the Rome Working Group recommends using the Bristol Stool Form Scale (BSFS) to identify IBS subtype.2
The US Food and Drug Administration (FDA) has recognized that a well-defined and reliable PRO instrument that measures the clinically important signs and symptoms associated with each IBS subtype would be the ideal efficacy assessment tool in clinical trials to assess treatment benefit.4 However, at this time such an instrument is not available. Although PRO instruments to measure signs and symptoms that would meet current standards5,6 are being developed for IBS, in its guidance for industry document, the FDA recommends assessment of the effects of treatment on abnormal defecation as an endpoint in IBS-D clinical trials, using the BSFS to capture stool consistency.4 The BSFS is a 7-point scale with descriptors ranging from “separate hard lumps, like nuts” to “watery, no solid pieces” that are usually used in conjunction with the Bristol Stool Chart (image scale).7,8
The BSFS was initially used in an epidemiologic stratified sample of approximately 72% of the residents of East Bristol, UK, from 1987 to 1989.9 The study was designed to assess the prevalence of gallstones, the population was heavily white, and older and younger patients were excluded. Patients were asked to rate their most common stool form based on a table describing 6 types of stool. Later research found the 7-point BSFS to be relevant for the assessment of stool transit time in 66 healthy volunteers.7 However, the BSFS was not developed using input from patients with IBS and there is no published information to indicate that it has been systematically validated to assess stool form in IBS. Importantly, a lack of published evidence suggests that its content validity (i.e., its adequacy to measure stool form and consistency in patients with IBS-D) has never been assessed in patients with IBS-D.
This report describes the development of a new measure to assess stool form and consistency in IBS-D, based on rigorous qualitative research methods. The impetus for developing this scale arose during a study to develop and validate a PRO instrument to assess the signs, symptoms, and severity of IBS-D (fully described elsewhere in the literature).10 During that process, qualitative assessment of the BSFS and a form of the BSFS adapted to measure diarrhea in patients with HIV11 (hereafter referred to as the adapted BSFS) was tested in the IBS-D population, and it was found that patients’ spontaneous descriptions of stool form and consistency did not correspond optimally with those of the 2 stool scales tested, and mostly widely used in this setting. Therefore, a decision was made to develop and cognitively test a new stool form and consistency scale applicable to IBS-D in the clinical setting, using the information provided by patients with IBS-D, input from clinicians, and the literature, to better meet the requirements for content validity as set forth in the FDA’s guidance for industry on PRO measures5 and IBS.4
Overall study design
Qualitative research was carried out over 4 separate phases enrolling a total of 113 patients with mild, moderate, or severe IBS-D, as defined by the Rome III diagnostic criteria.1 All phases of the study were performed in accordance with the ethical principles of the Declaration of Helsinki and are consistent with Good Clinical Practice and applicable regulatory requirements. All patients provided written informed consent prior to the start of the study. Briefly, the initial concept elicitation stage (phase 1) comprised 8 qualitative single-gender focus groups of two to nine patients per group, to obtain information from patients regarding their experiences with IBS-D (e.g., symptoms, effect on daily life) to inform the content and structure of the PRO.6,12
Based on the information provided, a PRO instrument comprising an IBS-D Daily Symptom Diary (referred to hereafter as the Diary) and an IBS-D Symptom Event Log (referred to hereafter as the Event Log), which included a stool form and consistency item, was developed to assess IBS-D symptoms. The understanding of the stool form and consistency items by patients with IBS-D was tested during phase 2 in 11 one-on-one cognitive interviews using a think aloud process.13,14 The Diary and Event Log were revised based on feedback from the cognitive interviews, additional focus groups of 16 patients (phase 3), and comments from the FDA. Face and content validity was tested using the May 2011 6-item IBS-D Daily Symptom Diary and the May 2011 4-item IBS-D Symptom Event Log in one-on-one cognitive interviews. After the first nine interviews, the PRO instrument was revised to the June 2011 6-item IBS-D Daily Symptom Diary and June 2011 4-item IBS-D Symptom Event Log, and these were cognitively tested with an additional 11 patients (phase 4). Different patients participated in each phase.
Qualitative assessment of the BSFS and adapted BSFS
In phase1, spontaneous reports from patients describing the consistency of their stools suggested that the BSFS did not have the granularity required to describe the diarrhea portion of the IBS-D continuum; therefore, the adapted BSFS11 was tested. In phase 2, this scale was cognitively debriefed with 11 patients and found to be interpretable, relevant, readable, and to have an acceptable format. When additional qualitative work was recommended by the FDA to clarify concepts, there was an opportunity to obtain patient feedback on the content of the adapted BSFS relative to the BSFS. Consequently, 2 sex-specific concept clarification focus groups in phase 3 included a debriefing of both the standard and adapted BSFS. Four documents showing stool image scales were provided: the adapted BSFS first without and then with written descriptors, and the BSFS first without and then with written descriptors. For the stool image scales without descriptors, patients were asked to write about and describe their own stool form and consistency, and then discuss descriptors of each image as well as the frequency with which they experienced each type of stool. Next, patients were shown the stool image scales with descriptors and asked if each caption was an appropriate representation of each of the images. Since both of the available and mostly widely used scales were found to be suboptimal, in that there were overlapping or imprecise descriptions, during this process, a new stool form scale applicable to IBS-D for both research and in the clinical setting was developed and cognitively tested.
Development of the new stool form scale
The process used to develop the new stool form scale, named the Astellas Stool Form Scale (ASFS), is shown in Figure 1. The verbal descriptors selected for the scale were obtained by comparing and contrasting spontaneous patient descriptions of their own stools (provided in focus groups conducted during phases 1 and 3) with the descriptions that patients had spontaneously provided (verbally and written on handouts) of the BFSF and adapted BSFS images (provided in focus groups conducted during phase 3). Descriptors were excluded from consideration if they were not saturated, too vague, triggers rather than true descriptors (e.g., “too much fried food”), or misinterpretations of the BSFS or adapted BSFS images. The final stool descriptors were selected based on frequency of mention and consensus from the study team (which included clinicians, methodologists, psychometricians, 2 qualitative researchers and their qualitative research team, and Astellas staff) regarding the extent to which each description was specific to an image type but also suitable for a new image.
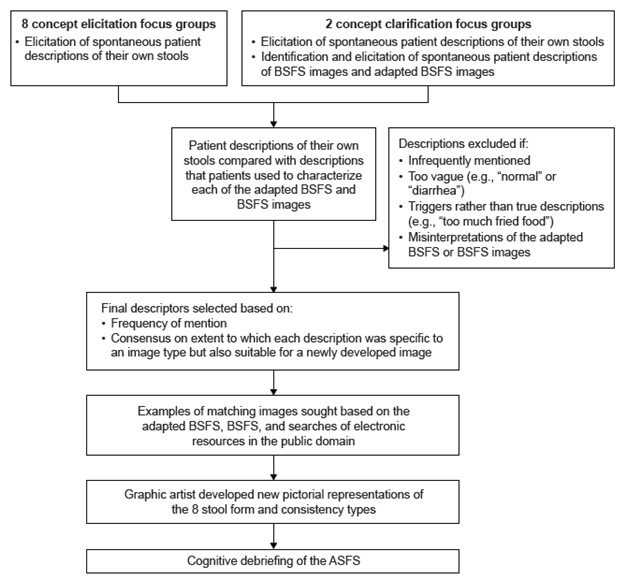
Figure 1 Process used to develop the new stool form scale ASFS: Astellas Stool Form Scale; BSFS: Bristol Stool Form Scale.
Based on these descriptors, examples of images matching the verbal descriptors were sought as potential models for new images. Sources included the standard and adapted BSFS images, the King’s Stool Chart, and electronic resources such as Google Images, PubMed, WebMD, HealthCentral.com, Centers for Disease Control and Prevention (CDC.gov), the Merck Manual, Medicine Online, and MayoClinic.com. A graphic artist developed a set of 8 original images specifically for this instrument using the final stool descriptors and example images from the literature. The understanding and comprehensiveness of the ASFS were then assessed in cognitive interviews (phase 4).
Patient information
Patient demographic and health information according to study phase is summarized in Table 1. Verbal descriptors of patients’ own stools were elicited from all phase 1 and 3 focus group participants (N = 50) and spontaneous descriptions of BSFS and adapted BSFS images from the phase 3 focus groups (N = 16). The new ASFS was assessed in 2 iterative sets of phase 4 cognitive interviews (N = 20).
Characteristic |
Phase 1 concept elicitation, N = 34 |
Phase 2 cognitive interviews, N = 11 |
Phase 3 concept clarification, N = 16 |
Phase 4 cognitive interviews, N = 20 |
Men, N (%) |
12 (35.3) |
5 (45.5) |
8 (50.0) |
9 (45.0) |
Women, N (%) |
22 (64.7) |
6 (54.5) |
8 (50.0) |
11 (55.0) |
Mean (range) age, y |
44.7 (23-69) |
50.7 (35-72) |
52.7 (40-68) |
49.0 (27-70) |
Race, N (%) |
|
|
|
|
Asian |
3 (8.8) |
1 (9.1) |
0 |
0 |
Black/African American |
4 (11.8) |
2 (18.2) |
4 (25.0) |
12 (60.0) |
White/Caucasian |
26 (76.5) |
6 (54.5) |
10 (62.5) |
9 (45.0) |
Hispanic/Latino ethnicity |
1 (2.9) |
1 (9.1) |
2 (12.5) |
0 |
Education, N (%) |
|
|
|
|
College, university, or graduate degree |
21 (61.8) |
0 |
12 (75.0) |
11 (55.0) |
Work status, N (%) |
|
|
|
|
Full-time |
15 (44.1) |
5 (45.5) |
13 (81.3) |
10 (50.0) |
Part-time |
7 (20.6) |
4 (36.4) |
2 (12.5) |
5 (25.0) |
Retired |
8 (23.5) |
1 (9.1) |
2 (12.5) |
1 (5.0) |
Health status, N (%) |
|
|
|
|
Excellent/very good/good |
27 (79.4) |
8 (72.7%) |
16 (100.0) |
16 (80.0) |
Fair |
5 (14.7) |
3 (27.3) |
0 |
4 (20.0) |
Poor |
2 (5.9) |
0 |
0 |
0 |
Severity of IBS-D (patient report), N (%) |
|
|
|
|
Very mild |
4 (11.8) |
1 (9.1) |
0 |
1 (5.0) |
Mild |
8 (23.5) |
4 (36.4) |
4 (25.0) |
3 (15.0) |
Moderate |
19 (55.9) |
4 (36.4) |
9 (56.3) |
13 (65.0) |
Severe |
3 (8.8) |
1 (9.1) |
3 (18.8) |
2 (10.0) |
Very severe |
0 |
1 (9.1) |
0 |
1 (5.0) |
Table 1 IBS-D, irritable bowel syndrome with diarrhea
aPatients whose feedback was used to develop and assess the new stool form scale
Comparison of the adapted BSFS with the BSFS
Detailed feedback from patients (N = 16) revealed that 2 of the images did not match their descriptors in the adapted BSFS and 4 of the images did not match their descriptors or were misinterpreted in the BSFS. Patients specifically noted that the descriptors did not match image 1 in both the BSFS (N = 3) and adapted BSFS (N = 5), and several patients noted that the descriptors did not match for image 2 (N = 4) or image 6 (N = 5) in the BSFS. For example, several participants looking at image 6 of the BSFS stated that “Number six, fluffy,” “Yeah fluffy doesn’t seem…,” and “Somehow that doesn’t fit” and that it was more like “mushy.” Another participant concurred, “Maybe mushy is better.” One participant described diarrhea as “Real liquidy, gooeyish, charcoaly-type” and another as “Sometimes it’s water…and then otherwise it’s just real soft coming out, but it’s not firm stool,” finding it difficult to match his experience with the images on the BSFS or adapted BSFS. Yet another patient, when asked, “and how often do you experience these?” responded emphatically, “I don’t experience any of those.” Five patients initially misinterpreted image 5 in the BSFS, and 3 patients misinterpreted image 4 in the adapted BSFS. In addition, the majority of patients (12/16) reported experiencing constipation, which is not represented in the range of stool forms in the adapted BSFS, and patients also indicated that there was inadequate coverage of diarrhea in the BSFS, based on the spectrum of the stool consistency they experienced.
Development and assessment of the new tool
For the first stage (phase 1) of development of the ASFS, stool form and consistency descriptors were grouped based on conceptual equivalence. Patient descriptors and corresponding BSFS and adapted BSFS images are shown in Figure 2. In contrast to the 7 and 6 descriptors, respectively, in the BSFS and adapted BSFS, the number of descriptors and stool images provided in the ASFS was increased from 6 to 8 to capture stool forms associated with constipation, ensure coverage of the broad spectrum of stool consistencies that patients had reported, and address the issues and gaps of the BSFS and adapted BSFS. The final ASFS (images with descriptors) and its instructions for use are shown in Figure 3, together with the BSFS and adapted BSFS for comparison.
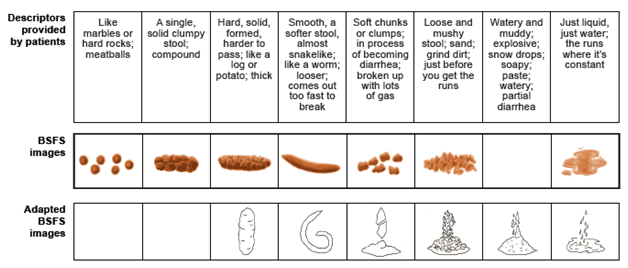
Figure 2 Stool descriptors spontaneously provided by patients, grouped by conceptual equivalence.
If the scale does not include the corresponding image, an empty box is shown. BSFS: Bristol Stool Form Scale. BSFS images reproduced from Heaton KW. Understanding your bowels: Family Doctor Publications; 1999, with permission from Family Doctor Publications. Adapted BSFS images reproduced with kind permission from Springer Science+Business Media [11], Figure 1, ã1995 Plenum Publishing Corporation.
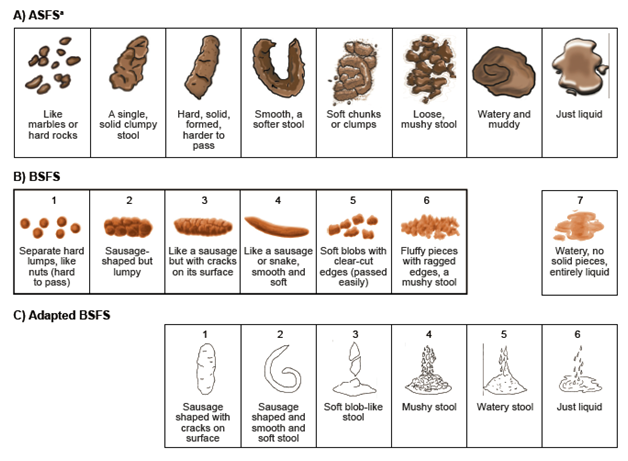
Figure 3 A) ASFS B) BSFS and Bristol Stool Chart [7,8] C) adapted BSFS [11].
aASFS instructions for use: “After every bowel movement, please fill out the date and time and answer the following question: Which best describes what your bowel movement looked like?”
ASFS: Astellas Stool Form Scale; BSFS: Bristol Stool Form Scale. ASFS ©2012 Astellas Pharma Global Development, Inc. (“APGD”). Reprinted in Health and Quality of Life Outcomes with permission of APGD. All rights reserved. To seek permission to reprint or distribute copies of the IBS-Daily Symptom Diary and/or IBS-D Symptom Event Log, e-mail copyright@astellas.com. BSFS stool chart reproduced from Heaton KW. Understanding your bowels: Family Doctor Publications; 1999, with permission from Family Doctor Publications. Adapted BSFS images reproduced with kind permission from Springer Science+Business Media [11], Figure 1, ã1995 Plenum Publishing Corporation.
In cognitive interviews, the majority of patients indicated that all the ASFS descriptors matched the images (N = 15/20; 75%), there were enough pictures to depict each type of bowel movement (N = 19/20; 95%), and all the images were clear (N = 14/20; 70%). Two (10.0%) were unclear about Image 5, and one of these patients (5.0%) was also unclear about Image 6. One patient each (5.0%) was unclear about Image 2, the difference between Images 6 and 7, and the difference between Images 7 and 8. The descriptor for ASFS image 4 was originally “smooth, a softer stool, almost snakelike”; however, based on feedback from 3 (15%) patients, the words “almost snakelike” were deleted.
If the scale does not include the corresponding image, an empty box is shown. BSFS: Bristol Stool Form Scale. BSFS images reproduced from Heaton KW. Understanding your bowels: Family Doctor Publications; 1999, with permission from Family Doctor Publications. Adapted BSFS images reproduced with kind permission from Springer Science+Business Media,11Figure 1, ã1995 Plenum Publishing Corporation.
Patient input is essential to the development of PRO measures.15 The BSFS was initially developed for use in an epidemiologic survey to assess the prevalence of gallstones in a mainly white population of residents aged 25 to 69 years in East Bristol, UK;9 the BSFS was only later found to have utility in predicting stool transit time in healthy volunteers.7 The adapted BSFS assessed in this study was designed for use in patients with HIV with diarrhea. During this study, feedback from patients with IBS-D indicated that the BSFS and adapted BSFS were suboptimal for use in this population. Some of the images were misinterpreted, and patients felt that some of the images matched neither the verbal descriptors nor their experience. In particular, the BSFS does not include a finely-graded diarrhea classification. The adapted BSFS includes a more finely-graded categorization of diarrhea than the BSFS, but does not include constipation stool forms. Because stool consistency of IBS can change over the course of the disease and with treatment, a preferable stool form scale would capture all stool types along the continuum of stool form and consistency experienced by patients in this population.
As a result of the inadequacy of the BSFS and adapted BSFS to assess stool consistency in the IBS-D patient population, the new ASFS stool form and consistency scale was developed. This expanded scale covers the broad spectrum of stool forms that patients reported and includes additional images to represent stools in patients with constipation as a symptom. Assessment of constipation is relevant in IBS-D because constipation occurs as a symptom in some patients; furthermore, the effects of treatment for IBS-D may result in constipation as an adverse event.2,16,17
Development and validation of a stool form scale specific to IBS-D are essential, as the use of a tool lacking validity in this patient population may lead to incorrect subtyping and inaccurate assessment of changes in stool characteristics in clinical studies, potentially resulting in imprecise evaluation of the efficacy and safety of treatments for IBS-D. This is particularly important because the BSFS is recommended by both the Rome Foundation2 and the FDA4 as an appropriate instrument to assess stool consistency and as a co-primary endpoint for IBS-D trials, despite the lack of published information to indicate that it has been examined on content validity in any of the IBS subtypes. A stool scale that is specific to IBS-D in the clinical setting also is valuable because individuals with IBS may be advised to adjust their treatment to achieve a stool consistency corresponding with a particular type on a stool form scale.18 The newly developed Astellas IBS-D Daily Symptom Diary and IBS-D Stool Form Scale jointly represent the first IBS-D qualitative symptom measure for evaluating treatment benefit developed in compliance with FDA regulatory guidance. The Stool Form Scale may be useful as a standalone assessment of stool form and consistency, not only for IBS-D, but for other patient population (e.g. IBS-C, or a measurement of diarrhea), similar to the wide utilization of both the BSFS and the adapted BSFS.8,11 Psychometric validation of the new ASFS and the results of quantitative testing of the new IBS-D PRO instrument for its reliability, validity, and ability to detect change to determine its suitability as a clinical trial endpoint will be reported in a separate publication. A limitation of this research is that it was exclusively conducted in patients from the United States, and it therefore awaits cross-cultural validation.
Compared with the BSFS, the new ASFS captures more of the diarrhea continuum and, in contrast to the adapted BSFS, it also captures the constipation portion of the stool consistency spectrum. Furthermore, the ASFS has the advantage of being developed with input from the actual patient population in which it is intended to be used. It was developed specifically for use as a tool for monitoring IBS-D symptoms and changes in symptoms over time to determine treatment benefit. Future assessment of this tool should focus on the clinical meaning of changes from one stool form/consistency to another on this more finely-graded scale. Additionally, the relationship between the new stool form and consistency grades and the thresholds for treatment response should be considered in light of the current recommendations in the FDA’s guidance on IBS regarding defining response.4 For example, the new ASFS category “soft chunks or clumps” may be a better threshold to define treatment response instead of “smooth, a softer stool,” which is more closely related to the threshold that is recommended in the current FDA guidance (BSFS image 4, “like a sausage or snake, smooth and soft”).4
In summary, this new stool form and consistency scale was developed using spontaneous patient descriptions, as well as feedback from patients on several versions of the tool, by means of rigorous qualitative data collection and analysis methods. During the cognitive interview process, the ASFS was well understood by patients and relevant to their experience of stool form and consistency in IBS-D; it was also deemed clinically relevant by clinicians, thus supporting the content validity of the ASFS. Although the ASFS shows promise as a tool to monitor change in patients with IBS-D and to assess treatments, its continued use requires demonstration of its reliability, validity, and utility in the clinical setting and as a tool to measure diarrhea in other patient populations.
We would like to thank the following Adelphi Values (formerly Mapi Values) staff members who participated in the data collection and analysis: Benjamin Banderas, Nina Galipeau, Allison Kornstein, Jessica Santiccioli, Jonathan Stokes, and Crystal Tellefsen; the following Astellas staff members who participated in the data collection, assessment, and analysis: Maggie Ayers, Jana Cummings, Allam Fakhoury, Amy Johnson, Rosanne Jordan, Maggie Liosatos, and Salim Mujais; and the graphic designer, Blake Waldman, who developed the images included in the ASFS. Writing assistance was provided by Helen Varley, PhD, CMPP, Tara Miller, PhD, CMPP, and Gill Sperrin, CBiol, MRSB, CMPP (medical writers at Envision Scientific Solutions).
Compliance with Ethical Standards
All phases of the study were performed in accordance with the ethical principles of the Declaration of Helsinki and are consistent with Good Clinical Practice and applicable regulatory requirements. All patients provided written informed consent prior to the start of the study, and Mapi Values obtained approval from an ethics board.
Declaration of Funding Interest
Writing assistance provided by Helen Varley, PhD, CMPP, Tara Miller, PhD, CMPP, and Gill Sperrin, CBiol, MRSB, CMPP of Envision Scientific Solutions was funded by Astellas. This study and the preparation of this paper were funded in full by Astellas.
Authors’ declaration of personal interests: KL, LTW, KR, and PM were employees of Adelphi Values (formerly Mapi Values) at the time of the study, which was contracted by Astellas to perform the research reported in this manuscript. LD-H, CL, IG, and BZ are employees of Astellas and GS and SK were employees of Astellas at the time the research was carried out. AJL has received consultancy fees from AstraZeneca, Ironwood/Forest, Prometheus, and Salix outside the submitted work.
KL assumed overall responsibility for the conduct of the study. KL, LD-H, LTW, KR, GS, AJL, PM, SK, CL, IG, and BZ contributed to the design of the study; KL, LTW, and SK performed the research and participated in data acquisition; all authors were involved in the analysis/interpretation of the data and drafting or critically reviewing/revising the manuscript; all authors approved the final version of the article.
None.

©2016 Lasch, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.