eISSN: 2373-6372


Review Article Volume 14 Issue 2
Department of Pharmacology, Medical Research and Clinical Studies Institute, National Research Centre, Egypt
Correspondence: Manal ME Ahmed, Department of Pharmacology, Medical Research and Clinical Studies Institute, National Research Centre, Giza, Egypt, Tel +20-10936-27027
Received: March 04, 2023 | Published: March 16, 2023
Citation: Ahmed MME. Contribution of the immune response to stroke provides promising directions for future stroke immunotherapies. Gastroenterol Hepatol Open Access. 2023;14(2):40-43. DOI: 10.15406/ghoa.2023.14.00543
In the entire world, stroke is a significant source of morbidity and mortality. Tissue plasminogen activator is the sole FDA-approved drug for the treatment of acute ischemic stroke at the moment. Despite the intensive search for new treatments, hundreds of drugs targeting various pathophysiological processes have failed clinical trials. The pathogenesis of risk factors, neurotoxicity, tissue remodelling, and healing are all stages of stroke that are impacted by the immune system. The brain and immune system communicate in both directions, and infection following a stroke is the main cause of patient mortality due to the immunosuppression it causes. While clinical sequelae like dementia may now potentially be described in terms of immunity, more recent research also suggests that the gut microbiota plays a role in the immunological response to stroke. Despite research indicating both harmful and positive actions, the precise roles of innate and adaptive components are still not well understood. If immunity and inflammation are neuroprotective or neurotoxic, time is a critical factor in determining this. In addition, it is evident that many suggested treatments are influenced by the local inflammatory environment. The most promising potential stroke immunotherapies are highlighted here as this review dissects the various elements of the immune response to stroke.
Keywords: stroke, immune response, acute ischemic stroke, immunotherapy, microbiota
AIS, acute ischemic stroke, CNS, central nervous system; ICAM-1, intracellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; DAMPs, damage-associated molecular patterns; NK, natural killer; SIID, stroke-induced immune depression; APCs, antigen-presenting cells
Stroke, a type of cerebrovascular disease that is 87% ischemic in nature, continues to be a leading cause of morbidity and mortality in the world. With nearly 100million disability-adjusted life years lost each year by survivors, it is currently the most prevalent source of acquired impairment.1 The only FDA-approved treatment for acute ischemic stroke (AIS) is intravenous recombinant tissue plasminogen activator (rtPA); nonetheless, despite the intensive search for new therapeutic approaches, the options for AIS treatment are few. The hypoperfused but potentially salvageable area known as the ischemic penumbra can be perfused again by tPA through thrombolysis, which also prevents cell death. However, tPA's advantages are outweighed by its hazards after a therapeutic window of 4.5hours, with the incidence of hemorrhagic transformation rising sharply.2 Intra-arterial thrombectomy using medical devices is one alternative to thrombolysis, although it has its own drawbacks and consequences.3
The main goal of recommended therapy for AIS during the past few decades has been neuroprotection. Nevertheless, the therapeutic graveyard that is the field of brain-protective drugs continues to be home to approximately 1000 distinct compounds that have been abandoned after numerous unsuccessful clinical trials.4 The immune response, however, plays a crucial and complex role in central nervous system (CNS) injury, according to both animal and human research, and new potential therapies have been proposed as a result, despite the poor clinical translation.
The complicated relationship between the immune system and CNS in stroke has been the subject of numerous reviews.5–9 To our knowledge, no one has, however, looked at the complete range of immunological targets and prospective treatments. Therefore, the purpose of this review is to explore both the most promising directions for future stroke immunotherapy as well as the immune system's role in the onset, development, and resolution of disease in stroke.
Acute ischemic stroke (AIS) triggered immune response at its onset
A series of processes involving the CNS, its vasculature, the blood, and lymphoid organs are triggered when a stroke begins after the commencement of cerebral ischemia. When an artery is blocked, hypoxia, the generation of reactive oxidative species, and changes in shear stress across the lumen wall occur, which are the first signs of an ischemic stroke.10,11 Microvascular occlusion is the outcome of the coagulation cascade being set off. The concurrent decrease in nitrous oxide's bioavailability worsens platelet aggregation (NO). Blood-brain barrier permeability is increased by the interaction of oxidative stress, inflammatory mediators (such as IL-1 and TNFα), downregulated endothelial junction proteins, and elevated leukocyte- or vascular-derived proteases.
When endothelial cells separate from the basement membrane as a result of these processes, a critical stage is achieved, which allows free water and serum to enter the brain without restriction, resulting in bleeding.6 Chemo-attractants and prostaglandins produced by endothelial cells further promote leukocyte entrance into the infarct site.6 The activation of high-avidity integrin molecules on leukocyte surfaces and the upregulated expression of corresponding ligands on endothelium, such as vascular cell adhesion molecule-1 (VCAM-1) and intracellular adhesion molecule-1 (ICAM-1), further encourage the infiltration of neutrophils, macrophages, and other leukocytes. Leukocyte activation results in the production of reactive oxidative species, proteolytic enzymes, leukotrienes, cytokines, and platelet-activating factor, all of which increase neurotoxicity by encouraging vasoconstriction and platelet aggregation.10 Activated macrophages release a variety of pro-inflammatory cytokines into the perivascular space, and mast cell degranulation results in the release of histamine, proteases, and TNFα, which further compromises the integrity of the blood-brain barrier. Ischemia affects the brain parenchyma even if all of these processes are taking place in the blood arteries and perivascular spaces. A reduction in ATP synthesis results from the acute lack of glucose and oxygen brought on by hypoperfusion. The death of neuronal cells is subsequently brought on by a cascade of related cytoplasmic and nuclear processes (bioenergetic failure, acidotoxicity, excitotoxicity, oxidative stress, and inflammation).11 A new stage of the inflammatory response is triggered by the release of danger/damage-associated molecular patterns (DAMPs) from dying and dead neurons.8 IL-1b and TNFα are produced when pattern- recognition receptors are activated. As a result, an inflammatory milieu with elevated levels of reactive oxidant species, granzyme, perforin, and IL-17 is created. 11
As early as 24hours after a stroke, the adaptive arm of the immune response is triggered. 8 Since mice lacking T cells displayed lower infarct size and improved functional outcome, it is commonly known that T cells have a detrimental effect during this early period of stroke.12 The fact that the return of T cells completely eliminates this neuroprotection supports the theory that T cells play a role in the pathophysiology of stroke. In the early stages of stroke, natural killer (NK) cells and specific CD4+ T-cell subtypes increase neurotoxicity in an antigen-independent manner, possibly by secreting multiple cytokines such IFN-γ and IL-17. 6 Further neuronal cytotoxicity is mediated by infiltrating γδ and CD8+ T lymphocytes. Contrarily, antigen-specific T cells don't become involved in the pathophysiology of stroke until much later. They take part in autoimmune or tolerance reactions in a way that largely depends on whether they are skewed toward a more harmful [T helper 1 (Th1), T helper 17 (Th17)] or protective [T helper 2 (Th2), T regulatory (Treg)] phenotype.9 In the weeks following the commencement of the disease, B cells can create antibodies against brain-derived chemicals, causing further neuronal damage and perhaps causing clinical stroke sequelae like dementia.13 Self-limiting at the initiation of inflammation gives chance for structural remodelling and functional reconfiguration. Three processes10 characterise the end of the acute phase: (1) removing tissue waste and dead cells, (2) development of an anti-inflammatory environment; and (3) generation of substances that promote survival.
Numerous cell types, ranging from immune cells to neurons and astrocytes, collaborate to carry out the processes of repair and reconstruction, which include neuronal sprouting, angiogenesis, neurogenesis, and changes to the cellular matrix. These cells work as a unit to create proteases and growth factors, which facilitate remodelling of the ischemic region (Figure 1).10
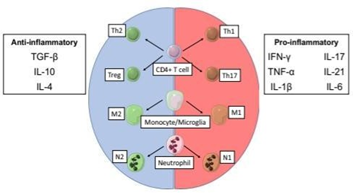
Figure 1 The effect of the inflammatory milieu on immune cell function in stroke. The balance between pro- and anti-inflammatory cytokines Influences the differentiation of activated microglia and leukocytes into neuroprotective (left) or harmful (right) phenotypes.20
Stroke impairs immunity
The immune system and central nervous system are in a reciprocal connection. Primary and secondary lymphoid organ activation results in a temporary 24-hour period of increased circulating levels of cytokines, chemokines, and proinflammatory mediators in the early stages of stroke, which is followed by a systemic inflammatory response. Two days after the stroke, a second phenomena known as stroke-induced immune depression (SIID) occurs in reaction to this initial inflammatory response. Lymphopenia, splenic atrophy, and elevated levels of anti- inflammatory cytokines are hallmarks of SIID. Through intricate humoral and neurological connections, including the sympathetic nervous system, the vagus nerve, and the hypothalamic-pituitary-adrenal axis, the CNS mechanically triggers these alterations in immune function. The level of brain damage does appear to be a key factor in SIID, while it is unclear if the location of the brain infarct affects the type of immune response that occurs after a stroke.15 According to extensive studies, pneumonia and urinary tract infections are the two most common post- stroke infections, affecting about 30% of stroke victims.16 Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Escherichia coli are the most often implicated bacteria. Nearly all occurrences of pneumonia are seen within a week, but only about half happen within the first two days following a stroke. In fact, a prospective study of stroke patients revealed that 75% of post-stroke infections were discovered within 3days of hospitalisation, pointing to a significant role for SIID rather than subpar patient care.16
Although SIID is harmful in terms of the post-stroke infections that may ensue, it may teleologically serve a protective purpose in which the peripheral immune response is downregulated to protect the CNS from autoimmunity. Breakdown of the blood-brain barrier during the acute stage of stroke provides immune cells practically unrestricted access to the CNS, and antigens often hidden in the brain become accessible, maybe for the first time. Within hours of an experimental stroke, it appears that these antigens can enter the bloodstream and be exposed to lymphocytes in lymph nodes, causing autoreactive T and B cells.17 While the phenomena of stroke-induced immunosuppression may be able to stop such autoimmune reactions, there are mitigating circumstances, such as infection, which aid to subvert it. There are two basic strategies for promoting autoreactive immune responses:8
Therefore, the overall effects of SIID are still unknown, and the final result will probably vary greatly amongst patients. On the other hand, there is no disputing the harmful impact of infections at all stages of stroke. As a result, methods that attempt to lower their prevalence (such as preventive antibiotics, hygiene measures, scoring systems for infection identification, suction or modified diets to minimise aspiration, and antiseptic-impregnated catheters) are certain to get more attention in the future (Figure 2).
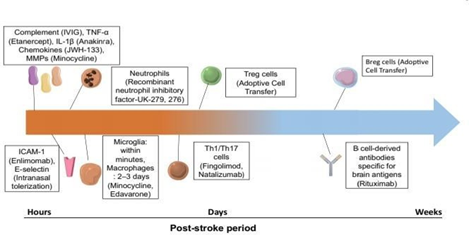
Figure 2 A time course of immune targets in stroke. Targets are placed according to the predominant role each plays in either neurotoxicity (hours to days post- stroke) or tissue remodeling and repair (weeks post-stroke).14
Stroke and gut microbial composition
An extensive and intricate immune system is present in the digestive system.9 Different signals from commensal bacteria can be transmitted to the host through a number of mechanisms by components of the gut-associated immune system. In this way, the immune system's maturation and subsequent homeostasis are significantly influenced by the gut microbiota. Recently, it has become clearer how the gut bacteria and the brain interact. Studies have revealed a connection, for instance, between commensal microorganisms and CNS autoimmune disorders.18 Importantly, dysbiosis of the gut microbiota may influence stroke and other acute brain injuries. In fact, it has been demonstrated in preclinical studies that changes in intestinal flora brought on by antibiotics affect both post-stroke neuroinflammation and the overall course of the stroke, with the same impact transferrable between mice via faecal transplant.19 Stroke may negatively impact the course of the disease by significantly altering the gut microbiota's composition. 20 After a stroke, there is a decrease in species diversity and an overpopulation of some phyla, including the Bacteroidetes. Through an abnormality in T-cell homeostasis, elevated levels of pro-inflammatory cytokines, and intestinal lymphocyte migration to the ischemic brain, this dysbiosis is hypothesised to affect immunological function.20–29 The brain-gut axis may influence disease outcome through a variety of other mechanisms, including the infiltration of activated intestinal monocytes into the infarct site in the acute phase of stroke. However, because our understanding of the gut microbiota and stroke itself is still limited, there may be many more such mechanisms.
This review throw more light on the importance of innate and adaptive immunity that show promise as therapeutic targets which also highlights a variety of likely prospective stroke immunotherapies.
None.
We declare there are no conflicts of interest.
None.

©2023 Ahmed. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.