eISSN: 2373-6372


Research Article Volume 14 Issue 6
1Department of Human Physiology, College of Medicine and Health Sciences, Gregory University Uturu, Abia state, P.M.B. 1012 Amaokwe Achara, Uturu, Abia State, Nigeria, 44110
2Human Physiology Department, Rivers State University, Port Harcourt, Nigeria
3Department of Biochemistry, Gregory University Uturu, Abia State, Nigeria
Correspondence: OM Onwuka, Department of Human Physiology, College of Medicine and Health Sciences, Gregory University Uturu, Abia state, P.M.B. 1012 Amaokwe Achara, Uturu, Abia State, Nigeria
Received: November 01, 2023 | Published: December 1, 2023
Citation: Onwuka OM, Nkpurukwe CI, Osuji AC. Comparative analysis of the impact of artificially and naturally ripened fruit on obesity-induced biochemical and hematological alterations. Gastroenterol Hepatol Open Access. 2023;14(6):175-179. DOI: 10.15406/ghoa.2023.14.00564
Aim: Comparative analysis of the impact of artificially and naturally ripened fruit on obesity-induced biochemical and hematological alterations was carried out.
Methodology: 4 g/kg of monosodium glutamate (MSG) was injected on neonates on day 2, 4, 6, 8, 10 to induced experimental obesity. 25g/kg of calcium carbide, CaC2 was used to artificially ripen fruits and naturally ripened fruit was also prepared. At age 7 months, Wistar rats induced with obesity were treated with artificially and naturally ripened fruits in their various groups for 28 days. Biochemical and hematological parameters related to oxidative stress, liver, kidney and blood functions were assayed.
Results: MSG-induced obesity significantly altered the bio-chemicals (MDA, SOD, CAT, GPx, AST, ALT, GGT, ALP, Urea, Creatine, K+, Na+, Cl-) and hematological parameters (RBC, PCV, Hb, WBC, WBC differential count) suggesting obesity could predispose to oxidative stress, liver, kidney and blood dysfunction; furthermore, naturally ripened fruit was able to significantly mitigate the effects exerted by obesity while artificially ripened enhanced the effects.
Conclusion: Fruit could be beneficial for obesity-induced biochemical and hematological alterations, but artificially ripened fruits could be deleterious to the stated condition. Hence, measures needs to be taken to ensure naturally ripened fruits are consumed since it can be beneficial to obesity-induced biochemical and hematological alterations as observed in this study.
Obesity is a pathophysiological state described by excess accumulation of body fat. It can be measured using Body Mass Index (BMI), which is expressed as kg/m2. A BMI of ≤ 30kg/m2 is considered obese.1,2 Obesity has been associated with increased risk of various health issues such as diabetes mellitus, hypertension, renal dysfunction, biochemical and hematological alterations.2,3
Several plants and fruits such as Camellia sinensis, cashew nut pawpaw, banana, pineapple, apple etc. have been essential in a balanced and healthy diet,2,4,5 and they can be beneficial for different pathophysiological conditions including obesity; since incorporating variety of fruits into diets can contribute to weight management and overall well-being.6,7 Banana; a popular and nutritious fruit reported to be beneficial for obesity, hematopoiesis and other biochemical activities is edible when ripened. Several ripening agents such as calcium carbide (CaC2), ethephon, were reported to be used for artificial ripening of fruits which in turn poses deleterious effect on the quality of the fruits and in turn affect the physiological system.8-10 Despites the nutritional value of fruits, the impact of artificially ripened fruit on obesity-induced physiological alterations is not fully elucidated. Hence, this study was aimed at evaluating the impact of artificially and naturally ripened fruit on obesity-induced biochemical and hematological alterations.
This study was performed in accordance to basic principles governing the use of laboratory animals. The animals were housed in tidy and cozy cages with alternating dark/light cycle and they had access to water and standard grower’s chow (Ladokun feed Ltd, Nigeria) ad libitum. Prior to experimentation, neonatal male Wistar rats gotten from animal house of Gregory University Uturu were group into five groups of 5 rats each.
Group 1 (control): 2ml normal saline
Group 2: obesity untreated; induced obesity with 4 g/kg of MSG and left untreated
Group 3: obesity treated with 500 mg/kg of metformin6
Group 4: obesity treated with 2ml of naturally ripened fruit
Group 5: obesity treated with 2ml of artificially (25g/kg of calcium carbide, CaC2) ripened fruit
The dose for metformin treatment was adopted,6 while the dose for CaC2 and methods of fruit ripening was adopted from.9,11 Obesity was induced using monosodium glutamate (MSG;12 the neonates were injected subcutaneously with 4 g/kg of MSG at days 2, 4, 6, 8, 10 and they were weaned at age 28days. Treatments as stated in experimental grouping were done orally at age 7 months for 28days.
After experimentation, blood samples were collected from the rats on anesthesia (sodium pentobarbital, 50mg/kg b.wt. i.p.) and used for biochemical and hematological assays. Biochemical parameters such as antioxidants (Malondialdehyde: MDA, Superoxide Dismutase: SOD, Catalase: CAT, Glutathione Peroxidase: GPx) was performed as described by,13 hepatic enzymes (Aspartate Aminotransferase: AST, Alanine Aminotransferase: ALT, Gamma-Glutamyl Transferase: GGT, Alkaline Phosphatase: ALP) as described by,14 Kidney function biomarkers (Urea, Creatine, K+, Na+, Cl-) as described by15 and hematological parameters (Red blood cell: RBC, Packed Cell Volume: PCV, Hemoglobin (Hb) concentration, White blood cell: WBC, WBC differential count) as described by11 Obesity was also ascertained using Lee index, body weight and size as described by16 Data obtained was statistically analyzed using GraphPad prism (v8); values were expressed as mean ± standard error of mean (SEM). ANOVA was performed between groups using Boneferroni post hoc comparison test and results were considered significance at P<0.05.
Studies reported that obesity induces biochemical and hematological alterations which is evidence in most pathophysiological conditions like renal impairment, cardiovascular impairment, liver damage, diabetes, hypertension etc.16-18 This study evaluated the biochemical and hematological responses in MSG-induced obesity treated with artificially (CaC2) and naturally ripened fruit (banana). Obesity index obtained showed it was significantly increased in Group 2 (obesity untreated) compared to treatment with metformin (Group 3) and naturally ripened fruit (Group 5) while there was non-significant impact of the artificially ripened fruit (Group 4) on obesity index when compared to obesity untreated and control (group 1), P<0.05 (Figure 1) which suggests that naturally ripened fruit followed the beneficial input of metformin in obesity condition as it significantly decreased body weight and Lee index when compared to obesity untreated (Group 2) which is line with studies that reported modulatory role of fruits like banana etc. on obesity.19,20 Artificially ripened fruit could not have any significant impact as it was ripened with calcium carbide which has been reported to compromise the nutritional quality of fruits21 which may have accounted for the inefficiency of the artificially ripened fruit observed.

Figure 1 Obesity index measured via body weight, nose-to-anus length and Lee index in MSG-induced obesity treated by artificially and naturally ripened fruit. **P<0.01, *P<0.05 compared to Group 1. ++P<0.01, +P<0.05 compared to Group 2. Group 1=control, Group 2=Obesity untreated, Group 3=obesity + metformin, Group 4= Obesity + naturally ripened fruit, Group 5=Obesity + artificially ripened fruit.
MDA, SOD, CAT, GPx are biochemical indicators of oxidative stress22 as expressed when there is increase in MDA which is formed during lipid peroxidation and decrease in SOD, CAT, GPx which are antioxidants that help to combat oxidative stress.23 In this study experimental obesity significantly increased oxidative stress when comparing Group 2 (obesity untreated) to Group 1 (control) at P<0.05; Treatment with metformin and naturally ripened fruit respectively was able to mitigate the obesity induced oxidative stress while artificially ripened fruit escalated the oxidative stress significantly (P<0.05) (Figure 2). The observed beneficial impact of naturally ripened fruit was in line with the study that reports its input in ameliorating oxidative stress,24 while the escalating oxidative impact mediated by artificially ripened fruits could be as result of the ripening agent (CaC2) which has been reported to induce oxidative stress.25
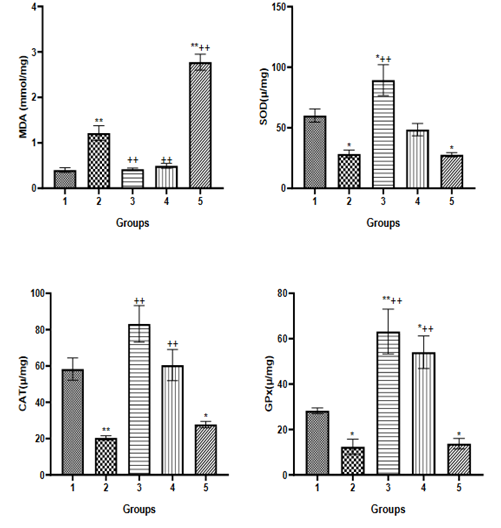
Figure 2 Biochemical indicators of oxidative stress (MDA, SOD, CAT, and GPx) in MSG-induced obesity treated by artificially and naturally ripened fruit. **P<0.01, *P<0.05 compared to Group 1. ++P<0.01, +P<0.05 compared to Group 2. Group 1=control, Group 2=Obesity untreated, Group 3=obesity + metformin, Group 4= Obesity + naturally ripened fruit, Group 5=Obesity + artificially ripened fruit.
Hepatic enzymes such as AST, ALT, GGT, and ALP are biochemical used to evaluate liver function or damage, they are elevated when there is liver dysfunction/damage; they can also be used to assess damage to other body organs such as heart, bile duct, bone etc.14 In this study, MSG-induced obesity significantly elevated AST, ALT, ALP and non-significantly increased GGT when compared to control (P<0.05); artificially ripened fruit escalated the increased AST, ALT, ALP and GGT significantly which suggest that artificially ripened fruit could predispose to liver damage in obesity condition as CaC2 used to induced fruit ripening has been reported to induce liver damage.26 Furthermore naturally ripened fruit was able to significantly (P<0.05) counteract the impact of MSG-induced obesity on hepatic enzymes (Figure 3).
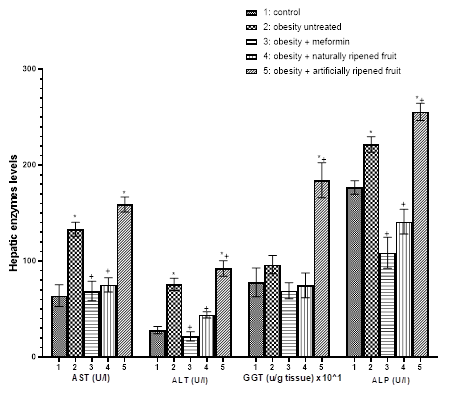
Figure 3 Hepatic enzymes (AST, ALT, GGT, and ALP) responses in MSG-induced obesity treated by artificially and naturally ripened fruit. *P<0.05 compared to Group 1. +P<0.05 compared to Group 2. Group 1=control, Group 2=Obesity untreated, Group 3=obesity + metformin, Group 4= Obesity + naturally ripened fruit, Group 5=Obesity + artificially ripened fruit.
Kidney function biomarkers (Urea, Creatine, K+, Na+, Cl-) are bio-chemicals use to evaluate physiological response of kidney which involves electrolyte balance, purification of blood and other homeostatic activities.27 Elevated levels of urea, creatine, K+, Na+, Cl- indicate kidney dysfunction; it was observed in this study that MSG-induced obesity significantly increased the levels of this kidney function biomarkers (Table 1) (P<0.05) which is in accordance to reports that obesity could predispose to kidney damage.15 Although naturally ripened fruit mitigated the impacts on kidney by significantly decreasing the levels of the biomarkers, artificially ripened fruit showed non-significant impact on obesity induced kidney dysfunction; but it was able to significantly increase the stated biomarkers when compared to control (P<0.05) (Table 1). This suggests that naturally ripened fruit could be beneficial to obesity-induced renal dysfunction while artificially ripened fruits could predispose to renal dysfunction.
|
Groups |
Urea |
Creatine |
K+ |
Na+ |
Cl- |
|
1 |
31.14 ± 1.96 |
0.30 ± 0.03 |
5.59 ±0.15 |
132.89 ± 2.21 |
97.16 ± 4.85 |
|
2 |
49.58 ± 2.81* |
0.52 ± 0.04* |
7.28 ±0.33* |
150.82 ± 4.57* |
110.55 ± 2.94* |
|
3 |
31.84 ± 3.55+ |
0.23 ± 0.02+ |
5.47 ±0.56+ |
130.16 ± 3.91+ |
96.19 ± 5.88+ |
|
4 |
32.54 ± 3.37+ |
0.31 ± 0.03+ |
5.92 ±0.24+ |
136.28 ± 2.27+ |
98.00 ± 6.28+ |
|
5 |
50.32 ± 2.55* |
0.59 ± 0.06* |
7.24 ±0.32* |
158.72 ± 5.80*+ |
112.10 ± 4.64* |
Table 1 Responses of kidney function biomarkers (Urea, Creatine, K+, Na+, Cl-) in MSG-induced obesity treated by artificially and naturally ripened fruit
*P<0.05 compared to Group 1. +P<0.05 compared to Group 2. Group 1 = control, Group 2 = Obesity untreated, Group 3 = obesity + metformin, Group 4 = Obesity + naturally ripened fruit, Group 5 = Obesity + artificially ripened fruit.
This study stated that MSG-induced obesity mediated hematological alteration by reducing RBC, PCV, Hemoglobin (Hb) concentration, WBC, WBC differential count (Table 2) which suggests that obesity could predispose to blood related diseases, altered body system protection and decreased inflammatory responses as the cellular components of blood (RBC, WBC, platelets etc.) know to perform this functions9,28 are deteriorated. Naturally ripened fruit ameliorated the impact mediated by obesity induced hematological alterations; furthermore artificially ripened fruit escalated the adverse impact exerted by the experimental obesity which is in accordance with reports suggesting that artificially ripened fruit can impair hematological variables via declined hematopoiesis while naturally ripened fruit enhances it8,9,11 which may have accounted for the impact of obesity on hematological parameters observed in this study (Table 2).
|
Groups |
1 |
2 |
3 |
4 |
5 |
|
RBC x (106/μl) |
5.77±0.28 |
2.69±0.23* |
6.73±0.33+ |
7.08±0.67*+ |
1.84±0.31* |
|
PCV (%) |
35.95±1.64 |
19.91±2.16* |
55.04±3.26*+ |
66.79±8.06*+ |
19.15±3.42* |
|
Hb (g/l) |
11.21±1.65 |
4.04±0.63* |
13.85±0.75*+ |
14.91±0.47*+ |
9.04±5.70* |
|
WBC x(105/μl) |
1.94±0.15 |
1.18±0.13* |
1.95±0.23+ |
2.16±0.19+ |
0.91±0.20*+ |
|
Lymphocytes x10 (%) |
67.28±3.54 |
14.13±1.77* |
75.93±5.38+ |
87.32±3.00*+ |
14.81±2.22* |
|
Monocytes (%) |
164.64±4.96 |
127.70±2.94* |
177.92±7.06+ |
167.28±11.89+ |
58.51±15.91*+ |
|
Neutrophils x10 (%) |
132.00±3.18 |
39.30±4.96* |
133.10±14.56+ |
140.30±26.01*+ |
39.80±4.59* |
|
Eosinophils (%) |
14.38±1.21 |
1.42±0.48* |
27.78±2.37*+ |
23.02± 3.02*+ |
5.60±1.75*+ |
Table 2 Responses of hematological parameters (RBC, PCV, Hb, WBC, WBC differential count) in MSG-induced obesity treated by artificially and naturally ripened fruit
*P<0.05 compared to Group 1. +P<0.05 compared to Group 2. Group 1 = control, Group 2 = Obesity untreated, Group 3 = obesity + metformin, Group 4 = Obesity + naturally ripened fruit, Group 5 = Obesity + artificially ripened fruit.
In summary, comparative analysis of the impact of artificially and naturally ripened fruit on obesity-induced biochemical and hematological alterations suggest that naturally ripened fruits could be beneficial for obesity-induced biochemical and hematological alterations which predisposes to obesity induced renal dysfunction, oxidative stress, liver damage, anemia etc.; furthermore artificially ripened fruit could escalate the deleterious effect exhibited by MSG-induced obesity. Hence, naturally ripened fruit is recommended for obesity and its associated pathophysiological conditions; while caution should be taken to avoid expose or ingestion of artificially ripened fruit. Fresh harvest is recommended as it can help cub and ensure non-exposure to artificially ripened fruits.
Naturally ripened fruit mitigated obesity-induced biochemical and hematological alterations, while artificially ripened fruits enhanced obesity-induced biochemical and hematological alterations forming basis for campaign against use of artificial methods like chemicals to ripen fruit as it can pose health hazard.
None.
The authors declare no conflicts of interest.

©2023 Onwuka, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.