eISSN: 2373-6372


Case Series Volume 13 Issue 5
National Medical Research Center of Coloproctology, Russia
Correspondence: Konovich EA, Ryzhikh National Medical Research Center of Coloproctology, Moscow, Russian
Received: August 22, 2022 | Published: September 30, 2022
Citation: Konovich EA, Khalif IL, Shapina MV, et al. Autoimmunity to extracellular matrix in inflammatory bowel disease: antibody to intestinal epithelial basement membrane and renal tubules in severe ulcerative colitis and crohn disease. Gastroenterol Hepatol Open Access. 2022;13(5):175-181. DOI: 10.15406/ghoa.2022.13.00519
Introduction (Background): Inflammation is accompanied by degradation and remodelling of extracellular matrix (ECM). Autoimmunity to the components of ECM, which develops at the same time, contributes to the intensification of inflammatory lesions.
Objective (Aim): To determine antibodies to ECM in patients with ulcerative colitis (UC) and Crohn's disease (CD).
Methods: Sera from 79 patients (UC-47 and CD-32) and 15 donors (controls) were examined by indirect immunofluorescence on monkey intestinal and kidney sections (Euroimmun, GERMANY).
Results: The frequency of IgG antibodies in UC, CD patients and controls was: 13/37 (35.1%), 10/28 (35.7%), 1/10 (10%) (p<0.05), and 16/43 (37.2%) (p<0.05), 2/16 (12.5%), 1/15 (6.6%) for intestinal mucosal ECM including epithelial basal membrane zone (EBM), renal tubules BM respectively; in UC patients with moderate to severe endoscopic activity antibodies were detected to intestinal EBM in 4/18 (22.2%) and 9/19 (47.3%) (p=0.05) cases and to renal tubular BM in 4/18 (22.2%) and 12/23 (52.1%) cases (p<0.05). In drug-resistant and drug-sensitive UC patients, antibodies to renal tubular BM were detected in 15/24 (62.5%) and 1/19 (5.2%) (p<0.05) cases, respectively. The number of immunopositive renal tubules increased significantly in reactions with sera from UC patients with severe versus moderate clinical (22.6% and 8.8%) and endoscopic (27.1% and 7.5%) activity and resistant versus sensitive patients to drug therapy (27.5% and 4.7%) ( all p<0.05). Antibodies to EBM were detected more frequently in CD patients with active intestinal inflammation (10/20) and strictures (8/10) than without (2/10, p<0.05). No antibodies were detected in CD patients with endoscopic remission (0/8), including those with strictures (0/5).
Conclusion: Antibodies to ECM structures (intestinal EBM and renal tubule EBM) in UC patients are significantly associated with severe colorectal inflammation and resistance to drug therapy. In CD, antibodies to EBM are present in patients with active intestinal inflammation combined with intestinal strictures.
Keywords: ulcerative colitis, crohn's disease, antibodies, extracellular matrix, epithelial basal membrane, intestinal strictures
UC, ulcerative colitis; CD, crohn's disease; IBD, inflammatory bowel disease; EBM, epithelial basal membrane; PBS, phosphate-buffered saline
Ulcerative colitis (UC) and Crohn's disease (CD) are the major clinical forms of inflammatory bowel disease (IBD). IBD develops in clinically immunocompetent individuals, has a chronic continuous or recurrent course, and presents with symptoms mediated by aggressive noninfectious inflammation. Total colorectal involvement is characterized by the presence of marked clinical, endoscopic and morphological signs with the development in some patients of resistance to anti-inflammatory chemo- and biological therapy and life-threatening complications, which dictate the need for surgical removal of the colon with subsequent significant reduction in quality of life. The pathogenesis of IBD includes a complex of pathogenic mechanisms, among which, according to most researchers, the loss of immune tolerance to obligate intestinal microflora is central. Multiple susceptibility genes, especially those responsible for defects in innate immunity in interaction with microflora pathogens, with the participation of environmental factors cause dysregulation of the immune system, leading to activation of cells and mediators of intestinal inflammation.1,2
Extraintestinal immune-mediated manifestations of various localizations, including liver, joints, skin, eyes, and other organs are observed in 20-40% of IBD patients. Primary cytokine dysregulation and autoimmune mechanisms are considered to be independent or contributing factors in the pathogenesis of inflammatory diseases accompanying IBD.3-6 Studies of susceptibility genes of IBD and IBD-associated primary sclerosing cholangitis and ankylosing spondylitis show the presence of multiple overlapping loci, which determine the formation of common pathways of immunopathogenesis.7
The extracellular matrix (ECM) forms the basis of the interstitial connective tissue, which supports the intraorganic architecture. The epithelial basal membrane (EBM) is a specialized form of the ECM that separates the epithelium from the surrounding stroma. ECM is a platform for cells, regulates intercellular contacts, cell proliferation, differentiation and migration, and performs signaling and transport functions.8
During inflammation there is a significant degradation of ECM, which is accompanied by the expression of new antigenic determinants.9. Antibodies to collagens and other ECM components are detected in patients with inflammatory diseases of various localizations.9–18 The presence of autoantibodies to ECM contributes to the aggravation of the underlying lesion and the development of inflammation in distant organs with more severe manifestations of disease.11,13-16,18–20
Destruction of ECM in inflammatory bowel diseases is due to significant expression of proteases that cause proteolysis of ECM and BM structural proteins and, as a consequence, an increase in epithelial and endothelial permeability and migration of immune cells into the mucosa.9 Destruction of ECM or, on the contrary, its excessive production and fibrous tissue mass increase are considered as morphological equivalents of penetrating and stricturizing types of CD, respectively.21 In UC, the production of collagen IV in the EBM of crypts and vascular endothelium BM and collagen V in mucosal ECM is increased.22 In the blood of UC and CD patients the level of markers of ECM components degradation (elastin, collagen V, vimentin) increases.23 In UC patients antibodies to collagens IV and I are revealed.24–26
CD and UC are observed in patients with autoimmune dermatoses that are mediated by autoantibodies to collagen types VII and XVII and other antigens of BM.27–29
Prolonged treatment with corticosteroids is usually associated with the development of resistant and dependent forms with a more aggressive and difficult to manage course.30 Many studies are aimed at finding available markers of inflammatory activity in the colon and predictors of response to drug and biological therapy.31,32 The above data suggest that involvement of ECM in the pathological process, accompanied by immunization of its components, contributes to severe colorectal damage and severe course of UC and CD.
Therefore, in the present study, we determined circulating antibodies to the intestinal and renal ECM of monkeys in UC and CD patients. Identified antibodies to the intestinal and renal tubular ECM area were analyzed in UC patients with moderate and severe clinical and endoscopic activity and resistant to drug therapy and in CD patients with intestinal strictures.
Patients
In the study we used serum of venous blood of 79 patients with IBD (UC-47 and CD-32), who were examined and treated at the department of inflammatory and functional diseases of the colon at State Scientific Center of Coloproctology named after A.N. Ryzhikh in Moscow (Russia). The diagnosis was made on the basis of clinical, endoscopic, radiological and ultrasound data and morphological examination of small and large intestine mucosa biopsy specimens.33 Age of UC patients was 35.5±1.7 (17-67) years. There were 25 men and 22 women. Mild form of disease was observed in 1 patient, moderate - in 16 patients, and severe - in 30 patients (fulminant - in 4 of them). Total involvement of the large intestine was diagnosed in 39 cases, left-sided colitis - in 6, proctosigmoiditis - in 2. Acute course of the disease was observed in 8 patients, chronic recurrent - in 14, chronic continuous - in 25 patients. Endoscopic activity of UC was determined by the severity of erosive-ulcerous lesions of mucosa.34 Minimal activity is characterized by the signs of mucous membrane inflammation: hyperemia, edema, friability, disorder of vascular pattern, minimal bleeding. Moderate activity is manifested by intensification of the above mentioned signs of inflammation with the presence of erosions and individual ulcers less than 5mm in size. At high activity there are multiple ulcers over 5mm in size, which merge and form extensive ulcerous defects. Minimal, moderate and severe endoscopic activity was revealed in 3, 21 and 23 patients, respectively. Hormone resistant form was observed in 14 patients, hormone dependent - in 8 patients. Toxic dilatation of the large intestine was observed in 4 patients, perforation of the ascending colon and bleeding from the large intestine - in 1 patient, pseudopolyposis - in 4 patients. Due to ineffectiveness of anti-inflammatory drug therapy 24 patients underwent colectomy for surgical treatment.
Age of CD patients was 31,4±2,1 (19-48)years. There were 21 women and 11 men. Colitis was observed in 16 patients, ileocolitis - in 12, terminal ileitis - in 4 patients. Total involvement of the large intestine was found in 16 patients, involvement of the left and right parts of the colon - in 5 and 3 patients respectively, proctosigmoiditis - in 4 patients. Active inflammatory process in the intestine was diagnosed in 24 patients, endoscopic remission - in 8 patients. Intestinal strictures were observed in 15 patients. Perianal complications (fistulas of rectum, fissure” anal canal) were found in 17 patients. Surgical treatment to remove the affected parts of the large intestine was performed in 9 patients.Blood serum of 15 healthy donors was examined as a control group.
Indirect immunofluorescence
Serum samples were stored before the study at -70ºC. The cryostat sections of intestines and kidneys of monkeys (Euroimmun kits, GERMANY) were incubated with 25µl of serum diluted 1:20 for 30min at room temperature. The sections were washed with phosphate-buffered saline (PBS) for 5 min and incubated with 20μl fluorescein-conjugated goat anti-human IgG antibodies (FITC-conjugated goat anti-human IgG) for 30min. After washing the section with PBS, the incubating medium (50% glycerol-PBS) and a coverslip were applied. Sera from a patient with Goodpasture's syndrome (antibodies to the kidney glomeruli) and a healthy person were used as positive and negative controls, respectively. The sections were examined using fluorescence microscopy (Jenalumar, Germany). 100 or more renal tubules were visualized on the kidney sections. The result was expressed as % of tubules with linear continuous fluorescence BM. Sera were considered seropositive if the number of immunoreactive tubules was higher than the cut-off (M±2σ) number in the control group, which was 15.0%.
Statistical analysis
Significance of differences in the proportions of patients with the presence of antibodies in the compared groups was performed using Fisher's exact test. Mann-Whitney U-criterion was used to compare the number of immunopositive renal tubules. Differences were considered significant at significance level p<0.05. Sensitivity, specificity, positive and negative diagnostic value of antibodies to renal tubules for detection of resistance to drug therapy in UC patients were determined. Statistica 10 software (Stat Soft Inc., USA) was used for data processing.
On sections of monkey intestine sera from UC and CD patients showed ECM luminescence of the lamina propria of the mucosa with spreading to the EBM zone of the villi and crypts (Figure 1A&B). Linear continuous fluorescence of renal tubule BM was observed on renal sections (Figure 1D). Antibodies to the EBM zone of the bowel were present in one-third of UC and CD patients. Antibodies to the renal tubule BM were also detected in a third of UC patients. In CD patients their frequency did not increase compared to the control group (Table 1).
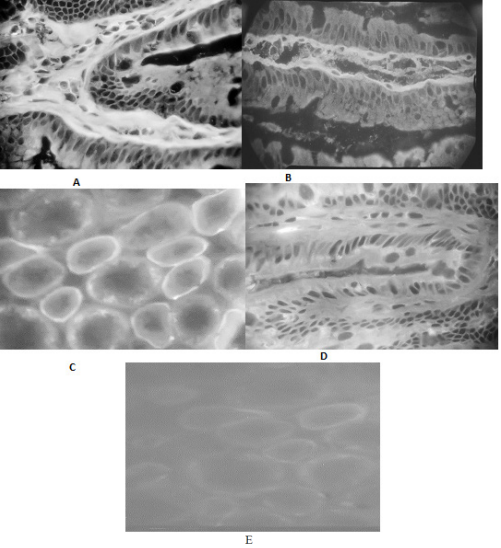
Figure 1 Indirect immunofluorescence on monkey intestinal and kidney sections with patient sera and fluorescein-conjugated goat anti-human IgG antibodies (FITC-conjugated goat anti-human IgG). Positive serum reaction of a patient with ulcerative colitis with ECM and EBM zone of the crypt in the intestinal mucosa plate (A) and with renal tubule BM (C). Positive serum reaction of a Crohn's disease patient with ECM and EBM zone in the villous mucosa (B). Negative reaction of donor serum (control) with mucosal crypt (D) and renal tubules (E). A,B,D - 660 x; c,e - 330 x.
Clinical groups |
Number of patients with antibodies/Number of patients in the group, abs (%) |
Ulcerative colitis |
13/37 (35,1)* 16/43 (37,2)*** |
Crohn's disease |
10/28 (35,7)* 2/16 (12,5) |
Control group |
1/10 (10,0) 1/15 ( 6,6) |
Table 1 Frequency of detection of serum antibodies to intestinal EBM area and renal tubule BM of the monkey in patients with ulcerative colitis and Crohn's disease
*- p<0.05 - ulcerative colitis and Crohn's disease vs (vs) control group; ** - p<0.05 - ulcerative colitis vs Crohn's disease;
Antibodies to intestinal EBM area in UC patients were detected twice as often in severe versus moderate endoscopic activity. In the groups of patients with moderate and severe clinical activity and with and without the effect of drug therapy, antibodies were detected with equal frequency (Table 2). The number of UC patients with moderate clinical and endoscopic activity with the presence of antibodies to renal tubular BM increased threefold compared to the control group, but the differences did not reach the level of statistical significance. The number of seropositive patients with severe clinical and endoscopic activity and resistant to drug therapy, as well as the number of renal tubules with which the sera of the patients of these groups reacted, significantly increased relative to the corresponding comparison and control groups (Table 3). The sensitivity and specificity of antibodies to renal tubule BM for detection of resistance to drug therapy were 62.5% and 94.7%, respectively. The positive diagnostic value was 93.7% and the negative diagnostic value was 66.6%.
Clinical groups |
The number of patients with the presence of antibodies/the number of patients in the group, abs (%) |
Clinical activity: Severe |
4/11 (36,3) 9/26 (34,6)* |
Endoscopic activity: moderate Severe
|
4/18 (22,2)** 9/19 (47,3) * |
With the effect of drug therapy Without the effect of drug therapy No drug effect therapy
|
6/16 (37.5)* 7/21 (33,3) |
Control group
|
1/10 (10,0) |
Table 2 Frequency of detection of antibodies to the EBM zone of monkey intestine in patients with ulcerative colitis depending on clinical and endoscopic activity and resistance to drug therapy
*- p <0.05 - groups with severe clinical and endoscopic activity and without the effect of drug therapy versus (vs) the control group; **-p = 0.05 - between groups with moderate and severe endoscopic activity
Clinical groups |
Number of patients with antibodies/number of patients |
Number of immunopositive tubules |
Clinical activity :Moderate Severe |
3/13 (23,0) 13/29 (44,8)* |
8,8 (0-38) 22,6 (0-95)* ** |
Endoscopic activity: Moderate Severe |
4/18 (22,2) 12/23 (52,1)* ** |
7,5 (0-38) 27,1 (0-95)* ** |
With the effect of Without the effect of drug therapy |
1/19 (5,2)
15/24 (62,5)* ** |
4,7 (0-21,8)
27,5 (0-95)* ** |
Control group |
1/15 (6,6) |
5,0 (0-15,5) |
Table 3 Frequency of detection of serum antibodies to BM of monkey kidney tubules and the number of immunopositive tubules depending on clinical and endoscopic activity and resistance to drug therapy of patients with ulcerative colitis
* P <0.05 - groups with severe clinical and endoscopic activity and no effect of drug therapy vs control group
** P<0.05 - groups with severe vs. moderate clinical and endoscopic activity and groups without effect vs. with effect of drug therapy
In half of CD patients with active intestinal inflammation, antibodies to monkey intestinal EBM zone were detected.
Their frequency in patients with active inflammation and intestinal strictures was significantly higher than in patients without strictures. No antibodies were detected in patients with endoscopic remission, regardless of the presence of strictures (Table 4).
Clinical groups |
Number of patients with antibodies / |
Crohn's disease: With active inflammation a) with the presence of strictures b) without strictures
With endoscopic a) with the presence of strictures |
10/28 (35,7)*
10/20 (50)** 8/10*** 2/10
0/8 0/5 0/3 |
Control group |
1/10 |
Table 4 Frequency of detection of serum antibodies to monkey intestinal EBM area in Crohn's disease patients with the presence of intestinal strictures
* p<0.05- Crohn's disease versus control group;
** p<0.05- active intestinal inflammation versus endoscopic remission;
*** p<0.05- active intestinal inflammation with and without strictures;
Our studies have shown that circulating antibodies to monkey intestinal mucosal ECM, predominantly to the EBM zone, are present in one third of UC and CD patients. In UC patients, antibodies to monkey renal tubule BM are detected with similar frequency. The affected colonic mucosa is a source of immunizing autoantigens and, most likely, the sera of UC patients reveal common antigenic determinants in the EBM of the colon and the BM of the renal tubules. The frequency of antibodies to renal tubules is not increased in CD patients compared with controls. These data suggest differences in the spectrum of immunizing colonic autoantigens in UC and CD, which is confirmed by a significant difference in IgG subclasses in the humoral immune response in UC and CD patients.2,35,36
Autoantibodies to BM components, particularly collagen and laminin, are major pathogens in a number of autoimmune diseases of the kidneys, skin, joints, lungs, and other tissues.27,37–39
Collagen IV is also present in EBM of the digestive tract, including the colon.40 Schmehl et al found a significant increase in the expression of collagen IV in the EBM of crypts and endothelial BM and collagen V in the ECM as the activity of inflammation increases.22 In an earlier study, we detected circulating antibodies to collagen IV in ELISA in 43.5% of UC patients and 16.6% of BC patients.24 The level of antibodies was higher in patients with severe resistant UC, which is confirmed in the present study that identified ECM as a target of autoantibodies.
The role of ECM components as autoantigens is consistent with the development of UC and CD in patients with autoimmune bullous dermatoses (autoimmune blistering diseases), which are mediated by autoantibodies to the BM of the squamous epithelium.27–29,44 In particular, CD is observed in patients with acquired bullous epidermolysis (epidermolysis bullosa acquisita-EBA), which is associated with antibodies to collagen VII in epidermal BM and colonic EBM.27–29,41,42.
In experimental EBA induced by administration of collagen VII to mice, skin, upper digestive tract and intestine lesions were observed in 100%, 60% and 30% of individuals, respectively.43
Local immune complexes in the colon wall of UC patients with severe inflammation and with extraintestinal manifestations, containing IgG1 and activated complement components, were found in studies performed in the 70-90s. The twentieth century.45–49
In separate studies, IgG antibodies to cytoskeleton proteins and EBM crypts adjacent to mucosal ulcers were isolated from the tissue of the affected colon.50–55 Inflammation is accompanied by an increase in the content of normal ECM components and their degraded fragments, the formation of an excess of modified peptides and the demasking of latent epitopes with new immunogenic determinants capable of causing an autoimmune response, which contributes to the maintenance and aggravation of the initial inflammation.9,56 In previous studies, we found that antigens localized in the EBM and the vascular wall of the mucosa of the normal colon, in UC, in addition to these structures are present in the intercellular fluid, which is accompanied by the formation of antibodies.57,58
We have previously determined that polyclonal immune rabbit antibodies prepared by immunization with mucosal supernatants from the colon of UC patients localized in indirect immunofluorescence EBM crypts and the vascular wall on sections of the affected colon and on sections of intact colon resected from cancer patients. By immunodiffusion in agar, the antigens were detected in the colon supernatants of UC patients, but were not detected in the supernatants of intact colon. We concluded that in destructive inflammation, antigens localized in the mucosal EBM accumulate in the interstitial fluid of the ECM and may be accessible to the cells of the immune system.57,58 Degradation of EBM is confirmed by a significant imbalance in the content of many proteins in the study of colonic mucosal proteome of UC patients.59 Changes in ECM composition and disruption of its three-dimensional structure lead to loss of connection of colonocytes and matrix, decreased migration, differentiation and regeneration of epithelial cells with subsequent destruction of crypts and formation of mucosal ulcers.22
In the present study, we found that the level of antibodies to intestinal EBM and renal tubule BM significantly increased in UC patients with severe endoscopic activity. Sera from these patients stained significantly more renal tubules on kidney section than sera from patients with moderate endoscopic activity and the control group. Thus, antibodies to ECM antigens are evidence of severe mucosal damage and, a possible pathogenic mechanism that enhances the initial inflammation in the colon.
Severe UC is associated with resistance to drug therapy.30 In a previous study in subgroups with a limited number of UC patients, we determined moderate sensitivity and high specificity of antibodies to renal tubular BM in drug-resistant patients.60 In the present work, the positive diagnostic value of this test is 94.7%. An extended prospective study is necessary to determine the predictive value of antibodies.
One of the severe manifestations of CD is the formation of intestinal strictures. Strictures develop due to excessive production of ECM by activated mesenchymal cells under the influence of multiple inflammatory mediators.61 The expression of collagens III and V is significantly increased in the intestinal wall.62 The development of strictures is combined with other syndromes caused by mutations of connective tissue protein genes, leading to the lesion of fibrogenesis and its mechanical properties.63–68 High joint mobility is observed in 70% of CD patients, which is the main symptom of a number of hereditary hypermobility syndromes.63 These syndromes are based on mutations in collagen V and tenascin-X genes, which cause disorders of fibrogenesis and mechanical properties of connective tissue.64–66 In CD patients with intestinal strictures, tenascin expression in small and large intestine submucosa and its level in blood are increased.67,68 In the present study, antibodies to intestinal EBM area in CD patients are detected significantly more often in active intestinal inflammation with strictures than in their absence. In contrast, antibodies are undetectable in patients in endoscopic remission, regardless of the presence of strictures. These data indicate that active intestinal inflammation is an initiating factor for the production of antibodies to EBM when the fibrous tissue mass of the strictures increases. A study of experimental models of inflammation-induced fibrosis reveals different kinetics of inflammatory and fibrogenic molecules and the subsequent development of fibrosis independent of inflammation.61,69 The absence of active intestinal inflammation leads to decreased immunization with ECM components.
Antibodies to collagens and other ECM components are detected in 20%-80% of patients with various inflammatory diseases, including arthritis, viral hepatitis, systemic vasculitis, autoimmune thyroiditis, chronic obstructive pulmonary disease, bronchial asthma, pulmonary fibrosis, periodontitis and others.9–18 Autoantibodies to ECM components, while not the primary pathogenic factor of these autoimmune and nonautoimmune inflammatory diseases, amplify the lesion in the diseased organ and may cause damage to distant organs and tissues.11,13–16,18–20
In support of this, we show that antibodies to ECM are detected in UC patients with severe resistant inflammation of the colon and in CD patients complicated by intestinal strictures.
Since ECM is a nonspecific structural basis of various organs, the study of its immunopathology seems promising in the search for common pathogenesis of intestinal inflammation and extraintestinal lesions in IBD.
In conclusion, in this study, we show that one-third of patients with inflammatory bowel disease show circulating IgG antibodies to ECM structures, including the EBM area of the bowel in UC and CD and the renal tubule BM in UC. In UC patients, antibodies to renal tubule BM are associated with severe clinical and endoscopic activity and resistance to drug therapy. In CD, antibodies to intestinal EBM are detected in patients with active inflammation combined with intestinal strictures.
None.
Author declares that there are no conflicts of interest.
None.

©2022 Konovich, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.