eISSN: 2373-6372


Research Article Volume 14 Issue 2
1Department of Pathology, Hospital of the Order of the Brothers of Saint John of God in Budapest, Hungary
2Department of Rheumatology, St. Margaret Clinic, Hungary
Correspondence: Miklós Bély M.D., Hospital of the Order of the Brothers of Saint John of God, Budapest, Hungary, Department of Pathology, H- 1027 Budapest, Frankel L. 54, Hungary, Tel (361) 438 8491, (06-30) 2194142
Received: March 04, 2023 | Published: March 17, 2023
Citation: Bély M, Apáthy A. Autoimmune and septic vasculitis in the gastrointestinal tract – A comparative postmortem study of 38 rheumatoid arthritis patients. Gastroenterol Hepatol Open Access. 2023;14(2):45-51. DOI: 10.15406/ghoa.2023.14.00544
Introduction: Systemic vasculitis of autoimmune origin (AV) is a basic manifestation of rheumatoid arthritis (RA). Different (acute, subacute, subchronic and chronic) stages of AV existing side by side in the same tissue sections demonstrate the relapsing nature of autoimmune vasculitis corresponding to the actual clinical activity of RA. Septic infection is one of the most serious complications of RA and can be accompanied with systemic septic vasculitis of recurrent nature (SV). Differentiation between AV and SV is of crucial importance because of different consequences and treatment. The aim of this study was to analyze the histological differences between AV and SV in blood vessels of the gastrointestinal (G-I) tract. Patients and methods: Gastrointestinal tissue samples of 33 RA patients with systemic vasculitis of autoimmune origin and 5 patients with systemic vasculitis of septic origin were analyzed histologically. Results: RA patients of both genders were affected by autoimmune or septic vasculitis at any time of the disease. Relapsing autoimmune vasculitis involved in different (nonspecific, fibrinoid necrotic or granulomatous) forms blood vessels of all sizes. Recurrent septic vasculitis involved arteries of all sizes in a nonspecific and fibrinoid necrotic form; the veins were intact. Granulomatous involvement of the veins was characteristic only for autoimmune vasculitis. Discussion and conclusion: Recognition and histological differential diagnosis of AV and SV are great challenges in surgical pathology especially in small biopsy specimens. The presence of a granulomatous type of vasculitis and involvement of the veins confirms histologically the autoimmune origin of this complication, while the lack of these supports a septic origin.
Keywords: Autoimmune (rheumatoid) vasculitis, septic vasculitis, gastrointestinal tract, histology
RA, Rheumatoid Arthritis; ACR, American College of Rheumatology; G-I, tract – gastrointestinal tract; HE, hematoxylin eosin staining; PAS, periodic acid schiff reaction
Prevalence of vasculitis – concerns the presence of inflammatory infiltration and structural changes in blood vessels of different calibers.
Severity of vasculitis – means different degrees of inflammatory infiltrates in segments or sectors of blood vessels. Severity of vasculitis was evaluated by semi-quantitative visual estimation on a 0 to 3 plus scale (based on the number of involved vessels/light microscopic field x40 of Olympus BX51).
Semi-objective score system of “severity”: “0” – no vasculitis, “1” – occasional blood vessels with vasculitis, “2” – less than 5 involved blood vessels, “3” – five or more involved blood vessels/microscopic field x40.
Remark: In case of medium size arteries or veins this corresponds to the absolute number of involved medium size vessels of a tissue sample, e.g.: “0” none, “1” only one “2” less than five, “3” 5 or more than five medium size vessels/tissue sample with a x20 objective lens.
Systemic vasculitis of autoimmune origin (AV) was defined as one of the basic manifestations of RA determined in 12 organs, excluding other causes of vasculitis, like hypertension, diabetes mellitus, tumors, septic infections, etc.
Systemic vasculitis of septic origin (SV) was ascertained as complication of generalized septic infection due to clinically identified bacterial agents.
Type of vasculitis
Ns – Nonspecific, Fn – Fibrinoid necrotic, Gr – Granulomatous
Stages of AV
“a” – acute, “b” – subacute, “c” – sub chronic, “d” – chronic stages, determined according to the inflammatory infiltration and structural changes of blood vessels of different calibers.
“a” – Polymorphonuclear neutrophilic leukocytes are present (with or without eosinophils) – “a” represents acute exacerbation of inflammation (acute stage) in combination with “b” or “c” and occasionally with “d”.
“b” – Characterized by intensive transmural inflammatory infiltration involving complete cross section of the blood vessels, dominated by T-lymphocytes with or without multinucleated giant cells and macrophages (histiocytes), and moderate structural changes (homogenization, hyalinization) of blood vessels.
“c” – More or less pronounced inflammatory infiltration dominated by T-lymphocytes (with or without macrophages) and pronounced structural changes.
“d” – Minimal or absent lymphocytic infiltration (without multinucleated giant cells and macrophages) without dominant structural changes of the blood vessels.
Size of blood vessels according to Szentágothai and Réthelyi (2002). Arteriole (a) – no internal or external elastic membrane, <500 micrometers in diameter. Small artery (A) – only internal elastic membrane present, vessels 500-1000 micrometers in diameter. Medium size artery (AA) – internal and external elastic membrane are present – vessel >1000 micrometers in diameter. Venule (v), small vein (V), medium size vein (VV) –accompanying (a), (A) or (AA).
Systemic vasculitis of autoimmune origin (AV) is a basic manifestation of rheumatoid arthritis (RA), and should be regarded as a “serious complication associated with significant mortality”.1 “Vasculitis itself is rheumatoid arthritis” (1986).2
Different (acute, subacute, subchronic and chronic) stages of AV existing side by side in the same histological section demonstrate the relapsing nature of autoimmune vasculitis, and corresponds to the actual clinical activity of RA.3–6
Generalized septic infection is one of the most important complications of RA. Septic infection can be accompanied with systemic septic vasculitis (SV), often as a recurrent process.3
Distinction between autoimmune and septic origin of vasculitis is of crucial importance, because of different consequences and treatment.3–6 Recognition and histological differential diagnosis of AV and SV are great challenges in surgical pathology especially in small biopsy specimens.
The aim of this study was to analyze the histological differences between AV and SV in blood vessels of the gastrointestinal (G-I) tract.
Patients (autopsy population) and methods
Gastrointestinal tissue samples from the autopsy of 33 RA patients with systemic vasculitis of autoimmune origin (AV) and 5 patients with systemic vasculitis of septic origin (SV) were histologically analyzed.
The extensive histological material and the available clinical and pathological reports were reviewed.
RA was confirmed clinically according to the criteria of the American College of Rheumatology (ACR).7 Systemic vasculitis was ascertained microscopically in agreement with the recommendations of the Consensus Conference (2013).8
The autoimmune or septic origin of systemic vasculitis (AV or SV) was stated according to the types of vasculitis, the size of involved blood vessels and the stages of vasculitis,3–6 clinically excluding other causes of systemic vasculitis, such as other autoimmune diseases (lupus, progressive systemic sclerosis, etc.), viral origin (hepatitis B and C), blood cancers, hypertension, endocrine diseases, reactions to certain drugs etc. The size” of arteries and veins was characterized according to the structure of the blood vessels (the „caliber” is fairly subjective and individual).9
The generalized septic infection was diagnosed at autopsy. The clinically or post mortem identified pathogenic agents and the strong (significant) positive correlation between septic infection and SV (association coefficient: 1, c2=26.1881, p<0.00007) supported the infectious origin of SV.4 Demographics of different patient cohorts were compared with the Student (Welch) t-probe.10
Demographics, onset and duration of RA patients with autoimmune and septic vasculitis of gastrointestinal tract are summarized in Table 1.
There was no significant difference in mean age at death, in onset or in duration of disease between female and male RA patients complicated with AV and SV.
Septic infection complicated by SV reduced the life expectancy of RA patients (62.00 years versus 67.18 years, p<0.332), which was especially pronounced in women (57.00 years versus 66.95 years, p<0.231), but these differences were not significant.
Disease duration of patients with SV was very short compared to the patients with AV (6.80 years versus 11.68 years, p<0.110), which was especially pronounced in women (5.33 years versus 10.63 years, p<0.164), but these differences were not significant (Tables 1 & 2).
Gender |
Number of autopsies |
Mean age in years at death |
Range |
Mean age at onset of disease |
Disease duration (in years) mean |
RA patients with AV |
33 |
67.18±10.64 |
32 – 83 |
56.94±14.63 |
11.68±10.34 |
Female |
20 |
66.95±11.11 |
32 – 82 |
59.47±10.15 |
10.63±7.46 |
Male |
13 |
67.54±9.86 |
53 – 83 |
52.92±19.07 |
13.33±13.55 |
RA patients with SV |
5 |
62.00±8.99 |
51 – 70 |
55.20±8.80 |
6.80±4.21 |
Female |
3 |
57.00±8.49 |
69 – 51 |
51.67±9.46 |
5.33±3.68 |
Male |
2 |
69.50±0.50 |
69 – 70 |
60.50±3.50 |
9.00±4.00 |
Table 1 Sex, mean age, range, onset and disease duration of RA patients complicated by AV and SV
RA, rheumatoid arthritis; AV, systemic vasculitis of autoimmune origin; SV, systemic vasculitis of septic origin; SD, standard deviation
RA patients n=161 (total) – “significant” p <0.05 |
Age |
Onset of disease |
Disease duration |
RA patients with AV n=33 versus pts. with SV n=5 |
p <0.332 |
p <0.745 |
p <0.110 |
Female n=20 of 33 versus n=3 of 5 |
p <0.231 |
p <0.365 |
p <0.164 |
Male n=13 of 33 versus n=2of 5 |
p <0.509 |
p <0.292 |
p <0.493 |
Table 2 Relationship between female and male RA patients with AV and with SV
Table 2 summarizes the statistical correlations (“p” values) between female and male RA patients with AV and with SV.
Prevalence (absolute number of involved vessels) and severity (densitiy) of vasculitis in 33 patients with AV and 5 patients with SV were different, but these differences (expressed in % of the total sum of AV or SV) were not significant, neither according to the type of vasculitis, nor according to the size of involved blood vessels (Table 3 and Figure 1A-D
N of RA patients |
Type of vasculitis |
Size of involved blood vessels |
Total |
|||||||
AV n=33 |
Ns |
Fn |
Gr |
a |
A |
AA |
v |
V |
VV |
|
AV Prevalence |
22 |
7 |
3 |
15 |
9 |
4 |
2 |
2 |
0 |
32 |
% |
68.75 |
21.875 |
9.375 |
46.88 |
28.135 |
12.50 |
6.25 |
6.25 |
0.0 |
100% |
AV Severity |
38 |
13 |
6 |
30 |
16 |
7 |
2 |
2 |
0 |
57 |
% |
66.67 |
22.81 |
10.53 |
52.63 |
28.07 |
12.28 |
3.51 |
3.51 |
0.0 |
100% |
SV n=5 |
|
|
|
|
|
|
|
|
|
|
SV Prevalence |
8 |
2 |
0 |
4 |
4 |
2 |
0 |
0 |
0 |
10 |
% |
80.00 |
20.00 |
0.0 |
40.00 |
40.00 |
20.00 |
0.0 |
0.0 |
0.0 |
100% |
SV Severity |
15 |
2 |
0 |
9 |
6 |
2 |
0 |
0 |
0 |
17 |
% |
88.24 |
11.76 |
0.0 |
52.94 |
35.29 |
11.76 |
0.0 |
0.0 |
0.0 |
100% |
Table 3 Prevalence and severity of AV and SV in blood vessels of the gastrointestinal tract (horizontal lines) arranged according to the type of vasculitis and size of blood vessels (vertical columns), (absolute number of involved vessels with vasculitis, and distribution expressed in % of total sum).
Prevalence and severity of AV and SV were runing parallel regarding the type of vasculitis or the size of the blood vessels (Table 3 and Figure 1A-D). The prevalence and severity of AV and SV expressed in % were nearly the same, the differences were not significant (Table 3).
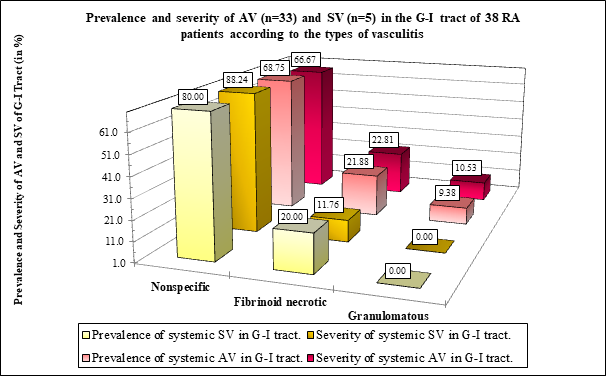
Figure 1B The prevalence and severity of AV or SV (expressed in %) was nearly the same comparing the nonspecific or fibrinoid necrotic vasculitis; the differences were not significant according to the type of vasculitis.
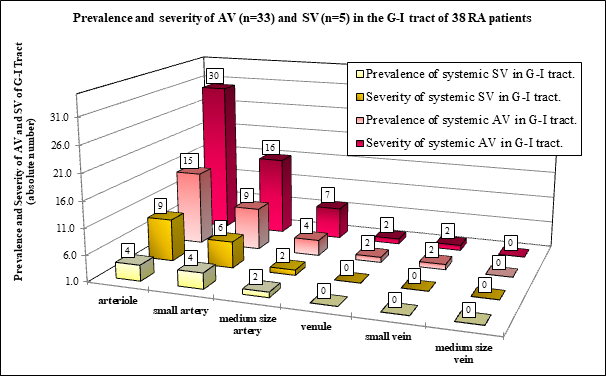
Figure 1C Absolute number of prevalence and values of severity according to the involved vessels in RA patients with AV or SV.
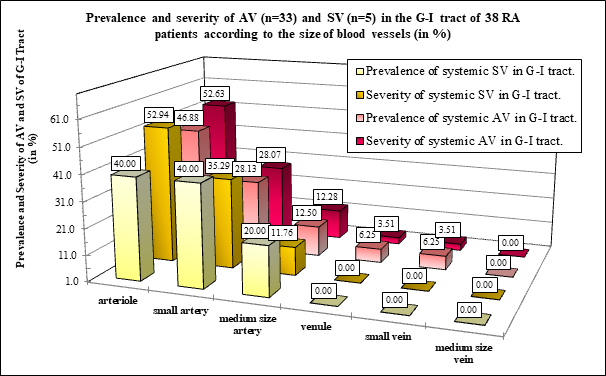
Figure 1D The prevalence and severity of AV and SV (expressed in %) was nearly the same in arterioles, small or medium size arteries; the differences were not significant (Table 4).
Three types of vasculitis were found in patients with AV: nonspecific (Ns), fibrinoid necrotic (Fn), and granulomatous (Gr). In patients with SV only nonspecific (Ns) and fibrinoid necrotic (Fn) types were detected; the Gr type was not. Different types of vasculitis existed side by side in the same histological section, indeed in different segments or sectors of the same blood vessel.
In the G-I tract the venules, small veins, and medium size veins were not involved by SV (n=0; 0.0 %); vasculitis of the veins was characteristic only for AV (Table 3 and Figure 3a-d).
Table 3 and Figures 1A-D summarize the prevalence and severity of AV and SV in the gastrointestinal tract according to the type of vasculitis and size of involved vessels.
Remarks to Table 1
“Prevalence" concerns the presence of vasculitis in different vessels (“yes” or “no”)
“Severity" means the number (density) of inflamed blood vessels per light microscopic fields x40.
There was no significant difference between prevalence and severity of vasculitis in 33 patients with AV and 5 patients with SV (Table 4).
Autoimmune vasculitis |
P< |
Prevalence |
|
AV Ns vs AV Fn |
0,030 |
AV Ns vs AV Gr |
0,006 |
AV Fn vs AV Gr |
0,223 |
Severity |
|
AV Ns vs AV Fn |
0,051 |
AV Ns vs AV Gr |
0,013 |
AV Fn vs AV Gr |
0,250 |
Septic vasculitis |
P < |
Prevalence |
|
SV Ns vs SV Fn |
0,052 |
SV Ns vs SV Gr |
0,017 |
SV Fn vs SV Gr |
0,187 |
Severity |
|
SV Ns vs SV Fn |
0,038 |
SV Ns vs SV Gr |
0,026 |
SV Fn vs SV Gr |
0,187 |
AV versus SV |
P < |
Prevalence |
|
AV Ns vs SV Ns |
0,073 |
AV Fn vs SV Fn |
0,337 |
AV Gr vs SV Gr |
0,162 |
Severity |
|
AV Ns vs SV Ns |
0,086 |
AV Fn vs SV Fn |
0,495 |
AV Gr vs SV Gr |
0,162 |
Table 4 Relationship between different type of vasculitis with AV and SV
Granulomatous type of vasculitis and involvement of the veins were characteristic to the autoimmune origin of vasculitis. Granulomatous type of vasculitis was not found in SV, and the veins were not involved by SV.
Figure 1A demonstrates the prevalence and severity AV and SV according to the type of vasculitis in 33 RA patients with autoimmune vasculitis, and in 5 RA patients with septic vasculitis. Absolute number of prevalence and values of severity of Ns vasculitis was higher compared to the Fn of Gr type of vasculitis in patients with AV or SV.
Granulomatous type of vasculitis was characteristic of the autoimmune origin of vasculitis; granulomatous type of vasculitis was not found in SV.
Figure 1B demonstrates the distribution of AV and SV expressed in % according to the type of vasculitis in 33 RA patients with autoimmune vasculitis, and in 5 RA patients with septic vasculitis. The prevalence and severity of Ns vasculitis was higher compared to the Fn of Gr type of vasculitis in patients with SV or AV; the “p” values of significance are summarized in Table 4. Granulomatous type of vasculitis was characteristic of the autoimmune origin of vasculitis; granulomatous type of vasculitis was not found in SV.
Figure 1C demonstrates the prevalence and severity of AV and SV according to the size of blood vessels in 33 RA patients with autoimmune vasculitis, and in 5 RA patients with septic vasculitis.
The prevalence and severity of AV or SV were more pronounced in arterioles than in small or medium size arteries. The veins were not involved by SV.
Figure 1D demonstrates the distribution of AV and SV expressed in % according to the size of blood vessels in 33 RA patients with autoimmune vasculitis, and in 5 RA patients with septic vasculitis.
The involvement of arterioles by AV or SV was relatively pronounced in contrast to the small or medium size arteries. Venules, small and medium size veins were not involved by SV.
The prevalence of Ns vasculitis in RA patients with AV was more common than in Fn (p <0.011) or Gr vasculitis (p <0.006), similarly to the severity of Ns vasculitis in Fn (p <0.025) or Gr (p <0.013) (Table 4 and Figure 2A & B).
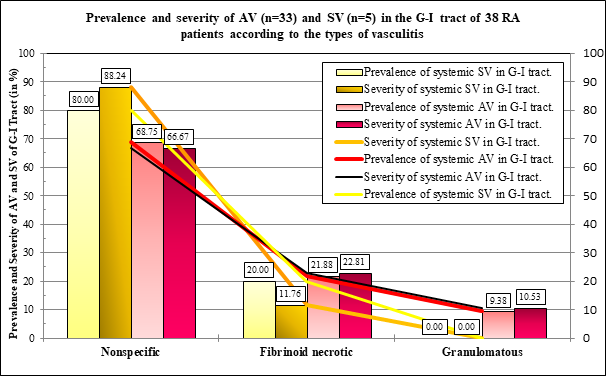
Figure 2A The differences (expressed in %) were not significant between prevalence and severity of AV and SV of the types of vasculitis. The prevalence and severity of AV and SV were runing parallel according to the types of vasculitis.
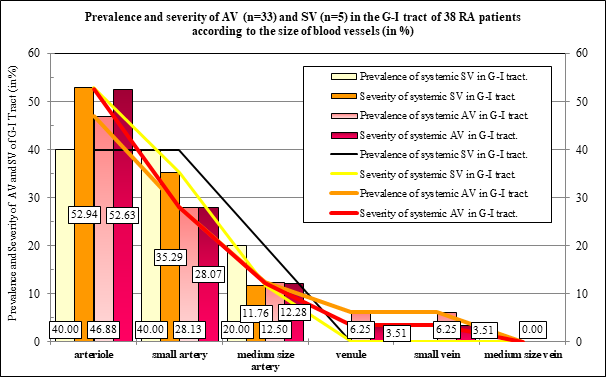
Figure 2B The differences (expressed in %) were not significant between prevalence and severity of AV and SV of the size of involved vessels. The prevalence and severity of AV and SV were runing parallel in blood vessels of a different caliber.
Gr type of vasculitis was not found in patients with SV; granulomatous vasculitis was characteristic only for autoimmune origin in the G-I tract.
Comparing different types of vasculitis in patients with AV and SV the differences in prevalence or in severity of vasculitis were not significant (Table 4).
Table 4 summarizes the statistical correlations (“p” values) between different types of vasculitis in RA patients with AV and SV (Figure 2A). Figures 3 & 4 show different types of AV in blood vessels of a different caliber.
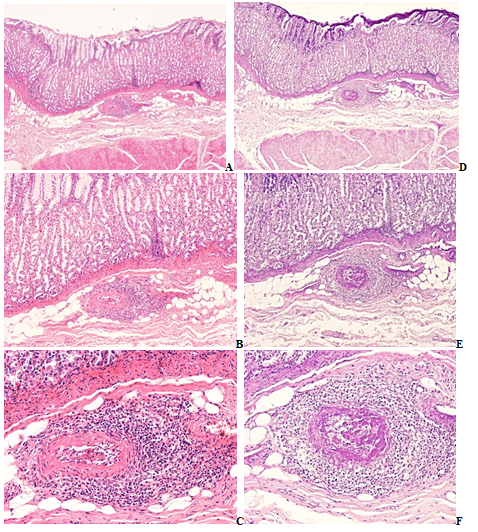
Figure 3A-F RA, stomach
a-c) Submucosal arteriole and venule, nonspecific, subacute-subchronic vasculitis of autoimmune origin, HE stain.
(d-f) Dieper section of the same tissue block, fibrinoid necrotic segment of the same submucosal arteriole and venule, PAS stain.
(a) Arteriole, HE, x 20, (b) Same as (a) x40, (c) Same as (a) x100,
(d) Fibrinoid necrosis in deeper segment of the same arteriole, same as (a) PAS, x20,
(e) Same as (d) x40, (f) Same as (d) x100.
.
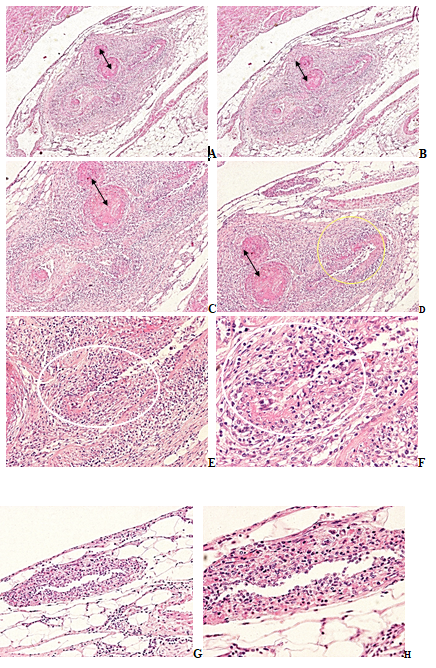
Figure 4A-H RA, duodenum, juntion of a small artery and arterioles with accompaniing venules and small veins, HE stain.
(a) Small artery and arterioles, thrombovasculitis, sectorial fibrinoid necrosis, nonspecific, subacute-subchronic vasculitis with moderate granulomatous transformation, accompanied with inflammation of venule and of small vein, HE, x20, (b) same as (a) HE, x20
(c) Same as (a) small artery and arterioles with nonspecific thrombovasculitis (arrows), sectorial fibrinoid necrosis (yellow ellipse), and moderate granulomatous transformation of the vessel wall (white ellipse), HE, x40, (d) Same as (c) HE, x40
(e) Same as (a) arteriole with moderate granulomatous transformation of the vessel wall (white ellipse), HE, x100, (f) Same as (e) x200,
(g) Same as (a) venule, non specific inflamatory infiltration in the vessel wall, HE, x100, (h) Same as (g) x200.
Original magnifications of all Figures correspond to the 24x36mm transparency slide; the correct height: width ratio is 2:3. The printed size may be different; therefore, the original magnifications are indicated (Figures 3 & 4).
Figure 5 shows hemorrhagic necrosis of colon due to nonspecific septic vasculitis in subchronic and chronic stages.
The clinical diagnosis of systemic vasculitis is mostly on visible skin involvement, e.g. urticaria, infiltrative erythema, petechiae, purpura, purpuric papules, haemorrhagic vesicles and bullae, nodules, livedo racemosa, deep (punched out) ulcers and/or digital gangrene.11
Contemporary studies recommend that ‘a definitive diagnosis of vasculitis should be made upon biopsy of involved tissue’.8 The high prevalence of vasculitis in skeletal muscles and peripheral nerves makes biopsy of the sural nerve (with surrounding muscle) a good target for the confirmation of suspected systemic vasculitis (with or without visible involvement of the skin).12
‘Based on histology, vasculitis can be classified on the size of the affected vessels and on the dominant inflammatory cell infiltration (neutrophilic, lymphocytic, eosinophilic etc.). Disruption of small vessels by inflammatory cells may cause fibrin deposition (saturation) within the lumen and/or vessel wall’.11 Vasculitic foci associated with extravascular granulomas (palisade of neutrophils and histiocytes) is a signal of enhanced risk.11
In present study the autoimmune or septic etiology of vasculitis was confirmed histologically according to the recommendations of the Consensus Conference (2013),8 Scott et al.,13 Schilling & Fassbender.14
Systemic AV and SV can complicate RA any time. The median age at presentation (63years) and the median duration of RA was (10.8 years) reported by Makol et al.1 and has essentially been the same in our autopsy patients.
In our autopsy population the AV or SV were present as relapsing or recurrent inflammatory processes involving blood vessels, characterized by different (acute, subacute, subchrinic and chronic) stages of vasculitis side by side in the same histological section, affecting blood vessels of different calibers. The veins of the gastrointestinal tract were involved only by AV; in patients with septic vasculitis the veins were intact.
Granulomatous type of vasculitis was found only in RA patients with AV. The presence of granulomatous vasculitis is diagnostic of its autoimmune origin and is an important histological sign in the differential diagnosis between AV and SV.
The histological characteristics of vasculitis or vascular changes help identifying autoimmune disorders in gastrointestinal tissue.
Histological analysis of gastrointestinal biopsies or surgical specimens is important in the recognition of systemic vasculitis. Nonspecific or fibrinoid necrotic vasculitis can complicate both autoimmune and septic vasculitis. The presence of granulomatous vasculitis confirms histologically the autoimmune origin of this complication; its absence supports septic origin. The involvement of the veins supports the autoimmune origin of vasculitis.
None.
The authors declare no conflict of interest.
None.

©2023 Bély, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.