eISSN: 2373-6372


Research Article Volume 2 Issue 2
1DG Health, Bangladesh
2Department of Clinical Pathology, BSMMU, Bangladesh
3Department of Hepatology, BSMMU, Bangladesh
4Department of Microbiology, Bangladesh University of Health Sciences (BUHS), Bangladesh
Correspondence: Mesbah Uddin Ahmed, MS in Microbiology, Bangladesh University of Health Sciences (BUHS), Dhaka, Bangladesh
Received: April 16, 2015 | Published: May 16, 2015
Citation: Rahman T, Islam S, Ferdoushi S, Mortaz RE, Bhuiyan MMA, et al. (2015) Association of Serum Cytokeratin-18 Fragment Concentration in Patients with Different Types of Nonalcoholic Fatty Liver Disease.Gastroenterol Hepatol Open Access 2(2):00037. DOI: 10.15406/ghoa.2015.02.00037
Nonalcoholic fatty liver disease (NAFLD) is one of the most common forms of chronic liver diseases worldwide. The prevalence of NAFLD was 15% in the Asian population. In a study, NASH was observed in 42.4% of NAFLD cases in Bangladesh. It is a serious clinical problem because of its worldwide distribution and potential adverse sequel. Cytokeratin-18 fragment is a marker of hepatocyte apoptosis. Measurement of serum cytokeratin-18 fragment concentration is a noninvasive, quick and simple procedure that can be carried out easily in peripheral hospital. It can be very much helpful for early detection and frequent monitoring of disease progression and response to therapy. This cross sectional study was conducted in the Department of Clinical Pathology in collaboration with Department of Hepatology and Department of Pathology, BSMMU, Dhaka from March 2014 to February 2015. Forty patients who fulfilled the criteria of NAFLD were determined in our study. The concentration of serum CK-18 fragment was measured to evaluate its association in different types of NAFLD by enzyme linked immune sorbent assay (ELISA). In this study, we observed that the serum CK-18 fragment concentration was increased in relation to Nonalcoholic fatty liver disease Activity Score (NAS) (r=+0.7134, P<0.05). To assess diagnostic performance of CK-18 fragment were used receiver-operator characteristic (ROC) curve. The area under ROC was 0.918 for identification of NASH. Using a cutoff value of 150 U/L the sensitivity and specificity of CK-18 fragment were 85.7% and 80.8% respectively. The positive predictive value, negative predictive value and accuracy of CK-18 fragment were 66.67%, 90.91% and 80.00% respectively. Our data revealed that serum CK-18 fragment was strongly associated with Nonalcoholic fatty liver disease Activity Score (NAS). Serum CK-18 fragment may be a useful noninvasive tool for assessing NASH in patients with NAFLD.
Keywords: cytokeratin-18 fragment, nonalcoholic fatty liver disease
ELISA, enzyme linked immune sorbent assay; NAFLD, nonalcoholic fatty liver disease; ROC Curve, receiver-operator characteristic curve; NASH, nonalcoholic steatohepatitis
Nonalcoholic fatty liver disease (NAFLD) is one of the most common forms of chronic liver diseases worldwide with prevalence of 15%-30%.1,2 This prevalence is increasing day by day.3 Histologically nonalcoholic fatty liver disease is classified into nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH). It is a dangerous condition and care should be taken for diagnosis of NASH as early as possible. Early detection of NASH allows initiation of medical intervention which may be capable of halting and reversing the process and improves most of the clinical outcome. This would in turn prevent progression to hepatic cirrhosis and thus lowers the rate of morbidity and mortality of the patients as well.4 There are many noninvasive and invasive markers for the diagnosis of NASH which distinguish it from simple steatosis. Till to date, the gold standard for the diagnosis of NASH is histopathological examination of liver tissue. It is the only reliable tool for distinguishing NASH from simple steatosis and grading and staging of the disease. But this facility is not available in primary centre in our country. There are several studies which suggested that serum cytokeratin-18 fragment concentration test is the most consistent single parameter for differentiating NASH from simple steatosis.5 From the study of Feldstein et al.1 the sensitivity and specificity of cytokeratin-18 fragment level test in NASH are approximately 80% and 90% respectively.1 It also correlates well with the severity of NASH.6 A study of Wieckowska et al.7 had established the high diagnostic accuracy of CK-18 fragment for discrimination between NASH and simple steatosis or normal liver with a sensitivity of 90.5% and specificity of 94%7. In another study of Ylmaz et al.4 it has been suggested that serum cytokeratin-18 fragment may help to identify the patients with NASH.5 They found the sensitivity and specificity CK-18 fragment for the diagnosis of NASH are 60% and 97.4% respectively. In our country there is a lack of clinical research regarding the use of CK-18 as a serum marker in NASH where serum cytokeratin-18 fragment concentration can be reflected in patients with NASH. In this context, this study aims to evaluate the association of the cytokeratin-18 fragment concentration in patients with different types of NAFLD especially NASH.
Study design
Cross sectional study
Duration of study
From March 2014 to February 2015
Place of study
This study was conducted at the Department of Clinical Pathology, Department of Hepatology and Department of Pathology, BSMMU, Shahbag, Dhaka.
Sample size
Total sample size was 40
Sampling Technique
Purposive sampling was done. As per inclusion criteria the patients were enrolled in this study. The patient’s clinical history and clinical data were recorded in a pre-designed data-sheet and kept until the end of the study. The whole procedure was explained to the patient and informed consent form was taken.
Data collection instrument
Data collection sheet and laboratory reports
Study population
Patients with NAFLD admitted in the Department of Hepatology in BSMMU. Total NAFLD patients were divided into three groups according to the NAFLD activity score (NAS); NASH Clinical Research Network histological scoring system8 which is based on histopathological examination.8 These groups are described below.
Group A → Non NASH which includes simple fatty liver disease patients. Here 8 patients were included.
Group B → Borderline patients. Here 18 patients were included and
Group C→ Definitive NASH patients. Here 14 patients were included.
Inclusion criteria
Exclusion Criteria
Laboratory assays
Specimen collection
Measurement of CK-18 fragment level
It was measured by Enzyme Linked Immuno Sorbent Assay (ELISA) based on double antibody sandwich technology (M30 Apoptosense® ELISA (PEVIVA®). Cut off value has been determined 150 U/L by using ROC curve.
For liver biopsy
Liver biopsy specimen were processed for histopathological examination and stained by Haematoxyline & Eosin and Masson’s Trichrome stain. H&E and Masson’s Trichome section slides were evaluated using NASH Clinical Research Network histological scoring system.
Data collection
Data were collected by a predesigned proforma. The blood samples were obtained from patients with NAFLD properly. Patient’s information was obtained through using patient’s information sheet (questionnaire, clinical findings, histopathological report and laboratory reports).
Data management
Data were managed by editing, clearing and analysis was done by using statistical package of social science SPSS (17.0).
Ethical consideration
Prior to the commencement of this study, the research protocol was approved by the Ethical Institutional Review Board (IRB) of BSMMU, Dhaka. The aims and objectives of the study along with its procedure, risks and benefits of this study were explained to the patient’s attendants in easily understandable local language. Then the Informed Consent Form was taken from each patient both in oral and written form. Strict confidentiality was maintained regarding the information of the patients during and after the study. Respondents were assured of the ethical use of their biological sample that they would bear no cost for the study and universal precaution would be maintained strictly.
Safety Precautions
Universal precaution was obtained. Gloves, lab coat, and safety glasses were worn when handling all human blood products and infectious viruses. Disposable plastic, glass, paper and gloves that contact blood were placed in a biohazard bag or discard after autoclaving. Disinfection of all working surface was done with 10% bleach solution. Other potentially contaminated materials were disposed in a biohazard bag. Other non-disposable materials were disinfected at the end of working day. Hands were washed thoroughly after removal of personal protective devices used in handling specimens and kit reagents.
Table 1 shows age distribution of the study patients according to groups of nonalcoholic fatty liver disease (NAFLD). Out of 40 patients, 8 (20%) belonged to Group A, 18 (45%) belonged to Group B, and 14 (35%) belonged to Group C. Maximum number of patients Group A (50%), Group B (61.1%) and Group C (50%) belonged to age Group 26-40 years. Distribution of age of the patients were statistically not significant (P>0.05). The mean ( ± SD) age of Group A patients was 42.63 ± 10.07 (range 30-56), Group B was 40.06 ± 7.88 (range 26-56) and Group C was 43.43 ± 8.97 (range 30-60) years. Comparison of mean (< ±SD) age between Group A & B, A & C, B & C was not statistically significant (P>0.05). Table 2 shows gender distribution of NAFLD patients. Gender distribution of overall patients showed 70% female patients and 30% male patients. Female predominance was observed in all study groups. Out of 8 patients of Group A, 3 (37.5%) were male and 5 (62.5%) were female; out of 18 patients of Group B, 4 (22.2%) were male and 14 (77.8%) were female; and out of 14 Group C patients, 5 (35.7%) were male and 9 (64.3%) were female. Gender distribution of patients according to type of NAFLD, statistically did not show significant variation (P>0.05). Table 3 showed CK-18 fragment level mean ( ± SD) in Group A, Group B and Group C patients, respectively, were 72.56 ± 39.67 (range 31.20 151.00), 118.01 ± 81.64 (range 53.10 397.80) and 348.81 ± 243.48 (range 93.70 983.70) U/L. Statistical comparison of mean (± SD) CK 18 values between Group A and B was not significant (P>0.05). But between Group A and Group C and also between Group B and Group C were significant (P<0.05).
Age (years) |
Group A No. (%) |
Group B No. (%) |
Group C No. (%) |
P value |
26-40 |
4 (50.0) |
11 (61.1) |
7 (50.0) |
|
41-50 |
2 (25.0) |
6 (33.3) |
5 (35.7) |
0.710ns |
51-60 |
2 (25.0) |
1 (5.6) |
2 (14.3) |
|
Total |
8 (20.0) |
18 (45.0) |
14 (35.0) |
|
Mean±SD |
42.63±10.07 |
40.06±7.88 |
|
0.492ns |
Range |
30.00‑56.00 |
|
||
Mean±SD |
42.63±10.07 |
|
43.43±8.97 |
0.836ns |
Range |
30.00‑56.00 |
|
30.00‑60.00 |
|
Mean±SD |
40.06±7.88 |
43.43±8.97 |
0.285ns |
|
Range |
26.00‑56.00 |
30.00‑60.00 |
||
Table 1 Age distribution of the study patients according to groups of nonalcoholic fatty liver disease (NAFLD) (n=40).
Gender |
Group A No. (%) |
Group B No. (%) |
Group C No. (%) |
P value |
Male |
3 (37.5) |
4 (22.2) |
5 (35.7) |
0.622ns |
Female |
5 (62.5) |
14 (77.8) |
9 (64.3) |
|
Total |
8 (20.0) |
18 (45.0) |
14(35.0) |
Table 2 Gender distribution of the study patients according to groups of NAFLD (n=40)
Chi square test, ns=not significant
CK-18 Fragment level |
Group A (n=18) |
Group B (n=18) |
Group C (n=14) |
P-value |
Mean±SD |
72.56±39.67 |
118.01±81.64 |
|
0.126s |
Range |
31.20‑151.00 |
53.10‑397.80 |
|
|
Mean±SD |
72.56±39.67 |
|
348.81±243.48 |
0.0001s |
Range |
31.20‑151.00 |
|
93.70‑983.70 |
|
Mean±SD |
118.01±81.64 |
348.81±243.48 |
0.0001s |
|
Range |
53.10‑397.80 |
93.70‑983.70 |
||
Table 3 Distribution of serum CK-18 fragment level of the study patients according to groups of NAFLD (n=40)
Mann Whitney test; significance level at P<0.05
In Figure1 Positive and highly significant correlation (r=+0.7134, P<0.001) was found between serumCK-18 fragment level and NAS. Serum CK-18 concentration was measured in 40 NAFLD patients and expressed as U/L. Types NAFLD was categorized by Nonalcoholic fatty liver disease Activity Score (NAS) which was expressed numerically (0-8). In Figure 2 serum CK-18 fragment levels of all patients with NAFLD (expressed in U/L) correlated with fibrosis score. Positive but not significant correlation (r=+0.113, P>0.05) was found between serum CK-18 fragment level and fibrosis score. Table 4 shows regression analysis which revealed that serum CK-18 fragment (P=0.003) was significantly associated with the presence of NASH. In Figure 3 the Receiver-operating characteristic (ROC) curve analysis suggested that a cutoff value of 150 U/L has the sensitivity (85.7%) and specificity (80.8%) for detecting patients with NASH with an area under the curve (AUC) of 0.918 (95% CI: 0.828-1.007), (P<0.05). In able 5 validity of CK 18 fragment level in detecting NASH was evaluated by calculating sensitivity, specificity, positive predictive value, negative predictive value and accuracy. Table X shows effectiveness of CK 18 in the diagnosis of NASH. CK 18 shows 85.71% sensitivity, 80.80% specificity, 66.67% positive predictive value, 90.91% negative predictive value and 80% accuracy.
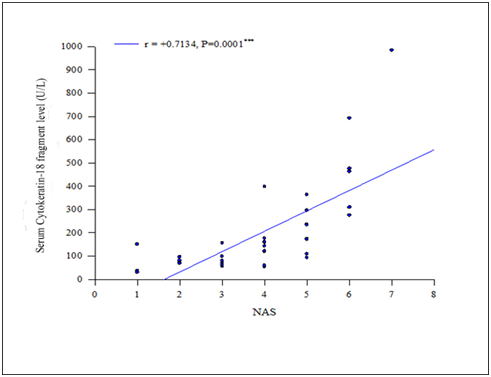
Figure 1 Showing positive correlation between cytokeratine-18 fragment level and NAS (n=40) (r = +0.7134, P<0.001).
Correlation between serum CK-18 fragment concentration and NAS (n=40)

Figure 2 Showing positive correlation between Serum CK-18 fragment level and fibrosis score (r = +0.113, P>0.05).
Correlation between serum CK-18 fragment concentration and Fibrosis score (n =40)
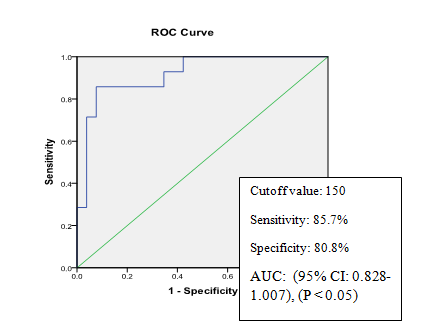
Figure 3Receiver-operating characteristic (ROC) curve of serum CK-18 fragment level for diagnosis of NASH (n=40).
B |
S.E. |
Wald |
Df |
Sig. |
Exp(B) |
|
CK-18 fragment |
0.02 |
0.007 |
8.679 |
1 |
0.003 |
1.021 |
Constant |
-6.342 |
1.987 |
10.185 |
1 |
0.001 |
0.002 |
Table 4 Regression analysis for serum CK-18 fragment for the diagnosis of NASH (n=40)
Parameters |
(%) |
Sensitivity |
85.71 |
Specificity |
80.8 |
Positive predictive value |
66.67 |
Negative predictive value |
90.91 |
Accuracy |
80 |
Table 5 Validity of CK‑18 fragment level in the detection of NASH (n=40)
This cross sectional study was conducted in the Department of Clinical Pathology in collaboration with Department of Hepatology, BSMMU, Dhaka. So far, any research was performed in Bangladesh evaluating the association of serum CK-18 fragment in patients with different types of NAFLD. Alam et al.9 found that NASH affects the population with mean age 40±9.7 with range from 30-50 of Bangladesh. Aktas et al.10 observed that the mean age of NAFLD patients was 47±12 (years) with range from 35-59 in that study. Papatheodoridis et al.11 showed that mean age of NASH patients in his study was 48±13 (35-61). Our study findings were similar with these studies. In the present study, serum CK-18 fragment was measured in different groups of NAFLD patients. In this study it has been found that CK-18 fragment levels were significantly higher in patients with NASH than the patients with simple fatty liver and borderline diagnosis. On the other hand, CK-18 fragment level did not differ significantly between the patients with simple fatty liver and borderline NASH. A similar study was done by Feldstein et al.1 where CK-18 fragment levels were significantly higher in patients with NASH than those with non NASH and borderline diagnosis. CK-18 fragment level did not differ significantly between the patients with simple fatty liver and borderline NASH also. Joka et al.12 also observed that, CK-18 fragment could make discrimination between simple fatty liver and NASH. In the study of Wieckowska et al.13 CK-18 fragment levels were significantly increased in the blood of patients with NASH compared with patients with simple steatosis.
There was strongly positive and significant correlation found between NAS and serum CK-18 fragment (r=+0.713 and P<0.05). Several studies found a significant positive correlation between serum CK-18 fragment levels and NAS.1,14,15 The relationship of fibrosis score with CK 18 was positive but not significant (r = +0.113, P>0.05). Tsutsui et al.15 also observed that there was no significant correlation between fibrosis and CK-18 fragment level. On the other hand, Joka et al.12 observed a positive significant correlation between CK-18 fragment level and fibrosis score12. However, our study finding was not consistent with the studies conducted by Joka et al.12 In our study, fibrosis was present in some patients with simple fatty liver while absent in some patients with NASH. It is the probable cause of contradiction regarding the studies described above. However, our study findings should be reconfirmed by further larger multicentred study. In our study regression analysis was performed to determine the independent predictor of NASH. It reveals that serum CK-18 fragment (P=0.003) was significantly associated with the presence of NASH. Several studies also observed that serum CK-18 fragment was independently associated with NASH.1,15 The area under the receiver-operating characteristic (ROC) curves for the diagnosis of NASH was depicted in our study. The Receiver-operating characteristic (ROC) curve analysis suggested that the area under the curve (AUC) for detecting the patients with NASH was 0.918. Wieckowska et al.13 described in their studies that the area under curve of CK-18 fragment for the detection of NASH was 0.93 which was nearly similar with present study. So our observation in this study was within international literature. The area under the ROC curve approach calculated the potential cutoff value to separate the patients with NASH from those with simple fatty liver or borderline diagnosis.
The Receiver-operating characteristic (ROC) curve analysis suggested that a cutoff value of 150 U/L has the sensitivity (85.7%) and specificity (80.8%) for detecting patients with NASH with an area under the curve (AUC) of 0.918. The study done by Feldstin et al.1 showed that the sensitivity and specificity of NASH were 80% and 90%. Papatheodoridis et al.11 the sensitivity and specificity of CK-18 fragment for diagnosis of NASH were 70% and 82% respectively. However, the test feasibility and accuracy of CK-18 fragment level should be reconfirmed by a larger multi-centered study with diverse population of patients in our country. Our study revealed that serum CK-18 fragment concentration is significantly higher in patients with nonalcoholic steatohepatitis. There is strong positive correlation between serum CK-18 fragment and Nonalcoholic fatty liver disease Activity Score (NAS). Our results indicate that serum CK-18 fragment showed good diagnostic performance for NASH. Liver biopsy is an invasive procedure for the diagnosis. Therefore, this study may help in early diagnosis and monitoring of NASH in NAFLD patients and would be beneficial for overall population.
Serum cytokeratin-18 fragment concentration has statistically significant positive relationship with different types of NAFLD patients. Besides this, CK-18 fragment concentration has significantly high sensitivity, specificity, negative predictive value and accuracy in assessing NASH. Serum CK-18 fragment can be used for the assessment of NASH in NAFLD patients. As the sensitivity of serum CK-18 fragment level was high, it can be used as a good screening tool for NASH which may help physicians to narrow down the patients population before biopsy. Serum CK-18 fragment can also be helpful for regular monitoring of the patients to see the disease progression and response to treatment. It might reduce the need for repeated liver biopsy of the patients and biopsy related hazards as well.
None.
The author declares there is no conflict of interest.

©2015 Rahman, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.