eISSN: 2373-6372


Research Article Volume 12 Issue 4
1Department of Medicine, Icahn School of Medicine at Mount Sinai, USA
2Department of Medicine, Baylor College of Medicine, USA
3Department of Digestive and Liver Diseases, Cedars-Sinai Medical Center, USA
4Department of Internal Medicine, Central Virginia VA Health Care System and Virginia Commonwealth University, USA
5Multiorgan Transplant Institute, Ochsner Health System, USA
6Department of Medicine, New York University Langone Medical Center, USA
7The Liver Institute, Methodist Health System, USA
8Department of Medicine, Northwestern University Feinberg School of Medicine, USA
9Department of Internal Medicine,Virginia Commonwealth University, USA
Correspondence: Amreen Dinani, Icahn School of Medicine at Mount Sinai, 1 Gustave L. Levy Pl., New York, USA, Tel 603-277-1445
Received: August 04, 2021 | Published: August 16, 2021
Citation: Dinani A, Sussman N, Noureddin M, et al. An algorithm for the management of non-alcoholic fatty liver disease in primary care. Gastroenterol Hepatol Open Access. 2021;12(4):114-122. DOI: 10.15406/ghoa.2021.12.00469
Background: Non-alcoholic fatty liver disease (NAFLD) encompasses a spectrum of conditions from simple hepatic steatosis to non-alcoholic steatohepatitis (NASH), a condition that includes fat accumulation, inflammation, and cell death. The single factor that predicts early death in patients with NASH is hepatic fibrosis. Hence, early identification and risk stratification of individuals with NASH and fibrosis is essential.
Methods: A panel comprising 11 liver disease specialists was assigned sections of the manuscript to present at a consensus meeting in December 2019. The goal was to develop a care pathway for primary care providers (PCPs) to identify patients at risk for NAFLD, stratify risk, and refer those in need of specialty services.
Results: We developed a simple algorithm to identify risk factors for NAFLD and recognize patients with progressive hepatic fibrosis. Patients with obesity, type 2 diabetes, abnormal liver tests, or incidental findings of hepatic steatosis should be evaluated for NAFLD, hepatic fibrosis and cardiovascular risk using family history and accepted calculators (FIB-4 and ACC/AHA). Risk stratification includes cardiovascular and hepatic complications. Patients with ≥ stage 2 fibrosis by non-invasive testing should be referred to hepatologists. We recommend lifestyle interventions and medical management of comorbidities for patients with NAFLD. Patients should be followed long-term with assessment of liver status every 6 months.
Conclusions: Using this algorithm in a primary care setting may raise awareness of risk factors for NAFLD, encourage timely lifestyle interventions, promote appropriate prescribing habits, result in more effective use of specialist consultations, and improve patient outcomes.
Keywords: fatty liver, steatosis, liver disease, fibrosis, cirrhosis, metabolic syndrome
AASLD, american association for the study of liver diseases; ACC/AHA, american college of cardiology/american heart association; ADA, american diabetes association; ALT, alanine aminotransferase; APRI, AST to platelet ratio index; AST, aspartate transaminase; AUDIT, alcohol use disorders identification test; BARD, BMI, AST/ALT ratio, diabetes; BMI, body mass index; EASD, european association for the study of diabetes; EASL, european association for the study of the liver; EASO, european association for the study of obesity; ELF, enhanced liver fibrosis; FIB-4, fibrosis-4 index; GLP-1, glucagon-like peptide-1; HbA1c, glycated haemoglobin A1c; HCC, hepatocellular carcinoma; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NFS, NAFLD fibrosis score; NIT, non-invasive test; PCP, primary care provider; SGLT2i, sodium-glucose co-transporter-2 inhibitor; T2DM, type 2 diabetes mellitus; VCTE, vibration-controlled transient elastography
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease and cirrhosis in the United States (US), affecting 25–33% of adults.1 The steady incline in NAFLD prevalence mirrors the growing obesity and type 2 diabetes mellitus (T2DM) disease burden. NAFLD is estimated to affect over one-third of the US population and over half of adults with T2DM.2,3 NAFLD is strongly associated with cardiovascular disease and non-hepatic cancers4,5 and recognized as a cause non-cirrhotic HCC.6 NAFLD has considerable burden on the US healthcare system accounting for ~8% of all-cause mortality and 36% of liver disease-related deaths.7
NAFLD encompasses a spectrum of conditions, ranging from simple hepatic steatosis (accumulation of fat in hepatocytes in the absence of hepatocyte inflammation)7,8 to non-alcoholic steatohepatitis (NASH), defined histologically by the presence of hepatic steatosis and hepatocyte inflammation7,8–10 with varying levels of fibrosis, that can progress to cirrhosis, and complications such as portal hypertension, liver failure, and hepatocellular carcinoma (HCC).11 Liver biopsy is currently the standard for the diagnosis of NASH and assessment of fibrosis;8,12 however, it is unappealing owing to several limitations, including pain, expense, and inaccuracy due to sampling errors.8,13
In this article, we present an algorithm for screening, risk stratification, and management of NAFLD patients in the primary care setting, based on expert opinion and consensus. The objectives of this algorithm are to help primary care providers (PCPs) identify patients at risk for NAFLD and recognize those who will benefit from specialist referral.
All 11 authors participated in a consensus meeting in December 2019 to develop a best-practices algorithm to identify, stratify, and manage NAFLD. All authors have expertise in evaluating and treating liver diseases, including NAFLD and NASH, and are advisors to NASHNET, a global Centers of Excellence Network committed to NASH care delivery innovation.14 Authors developed the preliminary content for the various sections, which was presented to the whole group for discussion and review. Through collaborative decision making, a consensus was reached on the algorithm, and recommendations are presented in this article.
Algorithm for screening, risk stratification, and management of NAFLD
Screening
|
Recommendation 1: Identify patients at risk for NAFLD based on the presence of ≥1 of the following: · Obesity, defined as body mass index (BMI) ≥30kg/m2 o Asian patients: ≥25kg/m2 · Increased waist circumference (≥35 inches for women, ≥40 inches for men) o Asian patients: >32 inches for women, >35 inches in men · Hyperglycaemia o T2DM or glycosylated haemoglobin ≥5.7% or fasting glucose ≥100mg/dL · Fatty liver on imaging |
NAFLD is under-diagnosed and under-reported in primary care.15 Obesity and T2DM are leading risk factors for NAFLD (Table 1).9,16 NAFLD, obesity, and T2DM are all independent risk factors for HCC.17–20 A systematic review and meta-analysis of 49,419 individuals with T2DM reported NAFLD in 56% of patients in the USA.2 The prevalence of NAFLD in obese patients undergoing bariatric surgery may be as high as 91%.21
|
NCEP ATP III (2004) |
IDF (2005) |
Definition of metabolic syndrome |
Presence of at least three of the five risk factors listed below |
Central obesity plus any two of the other four risk factors listed below |
Risk factors |
Defining level |
Defining level |
Central obesity |
Waist circumference
|
BMI >30kg/m2 or ethnicity-specific waist circumference
|
Raised blood pressure |
≥130/≥85mm Hg |
Systolic ≥130mm Hg or diastolic ≥85mm Hg |
Raised fasting plasma glucose |
≥110mg/dLa |
≥100 mg/dL |
Raised triglycerides |
≥150mg/dL |
≥150 mg/dL |
Reduced HDL cholesterol |
Men: <40mg/dL |
Men: <40mg/dL |
Table 1 Definitions of metabolic syndrome84,85
BMI, body mass index; HDL, high-density lipoprotein; IDF, International Diabetes Federation; NCEP ATP III, National Cholesterol Education Program Adult Treatment Panel III report; T2DM, type 2 diabetes mellitus.
aThe American Diabetes Association has established a cut point of ≥100mg/dL, above which persons have either prediabetes (impaired fasting glucose) or diabetes
There is a difference in opinion in who should be screened for NAFLD by multiple international guidelines. The European Association for the Study of the Liver (EASL), the European Association for the Study of Diabetes (EASD), and the European Association for the Study of Obesity (EASO) have jointly recommended that patients with obesity or metabolic syndrome should be screened routinely for NAFLD using liver enzymes and/or ultrasound.22 The American Diabetes Association (ADA) recommends that patients with T2DM or prediabetes and alanine aminotransferase. ALT) or fatty liver on ultrasound should be evaluated for presence of NASH and liver fibrosis.23 The American Association for the Study of Liver Diseases (AASLD) recommend screening for NAFLD in those with elevated aminotransferases and hepatic steatosis but no concrete recommendations are made for those with obesity or T2DM.8,24–26
We recommend that PCPs consider fatty liver in at-risk patients with three questions:
Our algorithm for screening, risk stratification, and management of NAFLD in the primary care setting is presented in Figure 1.
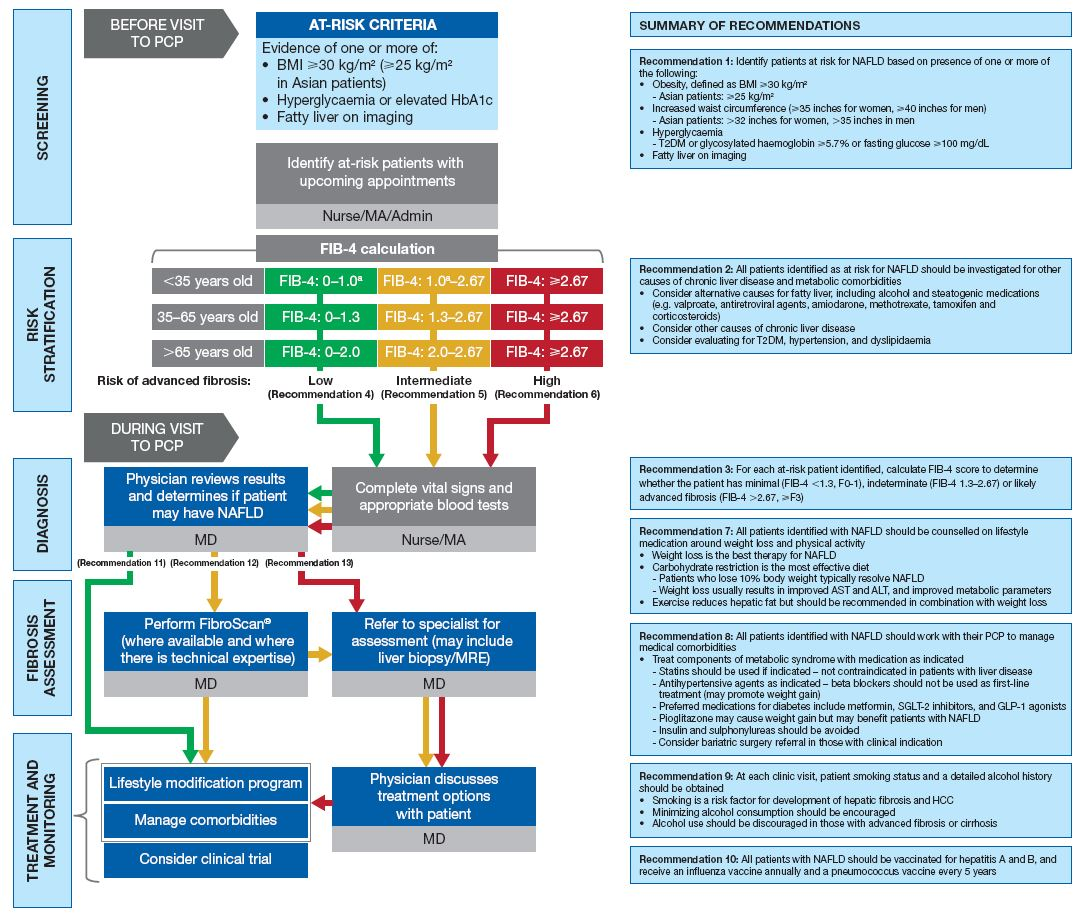
Figure 1 Algorithm for screening, risk stratification, and management of non-alcoholic fatty liver disease (NAFLD) in primary care.
Admin, administrative staff; ALT, alanine aminotransferase; AST, aspartate transaminase; BMI, body mass index; FIB-4, fibrosis-4 index; GLP-1, glucagon-like peptide-1; HCC, hepatocellular carcinoma; MA, medical assistant; MRE, magnetic resonance elastography; NAFLD, non-alcoholic fatty liver disease; PCP, primary care provider; SGLT-2, sodium-glucose co-transporter-2; T2DM, type 2 diabetes mellitus.
aExpert opinion – cutoff of 1.0 in individuals <35 years old not yet validated by clinical data.
Diagnosis
|
Recommendation 2: All patients identified as at-risk for NAFLD should be investigated for other causes of chronic liver diseases and metabolic comorbidities ·Consider alternative causes for fatty liver (Wilson’s disease ceruloplasmin level, chronic hepatitis C. especially genotype 3, genetic disorders of lipid metabolism) including alcohol and steatogenic medications (eg, valproate, antiretroviral agents, amiodarone, methotrexate, tamoxifen, and corticosteroids) · Consider other causes of chronic liver diseases o Haemochromatosis (ferritin and transferrin saturation) o Alpha-1 antitrypsin deficiency (alpha-1 antitrypsin levels) o Autoimmune liver diseases (antinuclear antibodies, antimitochondrial antibodies, anti-smooth muscle antibodies, and quantitative immunoglobulins) · Consider evaluating for type 2 diabetes mellitus, hypertension, and dyslipidaemia |
All patients identified as at-risk for NAFLD should have anthropometric measurements (height and weight to calculate BMI, a surrogate marker for obesity) vital signs, and laboratory tests performed (Table 2).8,27–36 Vital signs relevant to NAFLD include elevated blood pressure, indicative of essential hypertension, and increased waist circumference.
|
Test |
Rationale; normal valuesa |
|
Liver panel |
|
|
ALT |
Elevated ALT may indicate hepatocyte injury27b Normal range28: ≤35 U/L for men and ≤25 U/L for women |
|
Albumin |
Albumin is produced only in the liver, so low albumin may indicate impaired synthetic liver function27 Normal range29: 3.5–5.4g/dL |
|
Bilirubin (direct and total) |
High direct bilirubin may indicate liver dysfunction27 Direct bilirubin normal range29: 0–0.3mg/dL Total bilirubin normal range29: 0.3–1.2mg/dL |
|
GGT |
Elevated GGT is a predictor of liver mortality27 Normal range29: 8–78U/L |
|
BMP |
|
|
Serum creatinine |
Renal dysfunction is common in patients with cirrhosis, but also seen in patients with metabolic syndrome without NAFLD30 Normal range29: 0.7–1.3 mg/dL |
|
Serum sodium |
Hyponatraemia has prognostic significance in patients with cirrhosis31 Normal range29: 136–145 mEq/L |
|
INR |
Important indicator of liver function as clotting factors are made by the liver; significant liver injury may result in coagulopathy27 Normal range32: 0.8–1.1 |
|
CBC |
|
|
Platelet count |
Low platelet count may indicate portal hypertension, which occurs in advanced fibrosis31 Normal rang33: 150–400 ×109/L |
|
HbA1c |
Test for T2DM (component of metabolic syndrome); routine health parameter8 Normal31: <5.7% Prediabetes34: 5.7–6.4% |
|
TSH |
Test for hypothyroidism; routine health parameter8; low free T4 level is a risk factor for NAFLD35 Normal TSH range29: 0.5–5.0mIU/L |
|
Lipid panel |
Component of metabolic syndrome; routine health parameter8 |
|
HBsAg, anti-HBc, IgG (not IgM), anti-HBs, and anti-HCV with reflex to PCR |
To rule out viral hepatitis27 |
Table 2 Routine blood tests, with rationale
ALT, alanine aminotransferase; anti-HBc, total hepatitis B core antibody; anti-HBs, hepatitis B surface antibody; BMP, basic metabolic panel; CBC, complete blood count; GGT, gamma-glutamyltransferase; HbA1c, glycated haemoglobin A1c; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; Ig, immunoglobulin; INR, international normalized ratio; NAFLD, non-alcoholic fatty liver disease; PCR, polymerase chain reaction; T2DM, type 2 diabetes mellitus; TSH, thyroid-stimulating hormone
aNormal values vary between laboratories and may also differ between men and women and between adults and children. bALT may be within normal limits in patients with NAFLD36
There is no specific sign or symptom for NAFLD. In addition, there is no diagnostic laboratory test to confirm diagnosis. ALT can be normal in up to 50% of persons with NAFLD.37 While elevated ALT and aspartate transaminase (AST) levels indicate the presence of liver injury and NASH they do not always correlate with the severity of liver damage.27,38,39 An upper limit of normal for ALT of 35 U/L for men and 25U/L for women is recommended to guide management decisions.28 Low platelet count may indicate presence of portal hypertension. Albumin, total bilirubin, and international normalized ratio are indicators of liver synthetic function. Patients with cirrhosis frequently have more than one abnormality on routine blood tests.27
When assessing for NAFLD, exclude other causes of hepatic steatosis or chronic liver disease. Alcohol-related liver disease is the other common cause of fatty liver disease. Patients should be asked directly about their alcohol intake, for example, with the Alcohol Use Disorders Identification Test (AUDIT).40 As patients may under-report their alcohol consumption, assessment using biomarkers of alcohol use, such as ethyl glucuronide or phosphatidyl ethanol, may be useful.41 An AST level greater than ALT may be more indicative of alcoholic versus non-alcoholic steatohepatitis, except in patients with advanced liver disease. Although NAFLD indicates the lack of evidence for ongoing or recent consumption of significant amounts of alcohol, the precise definition of significant alcohol consumption in patients with suspected NAFLD is uncertain.8 Other causes of hepatic steatosis should be considered (Recommendation 2)8,13,27,42 and risk factors of metabolic syndrome should also be investigated (Table 3).
Test |
Components |
Cutoffs |
Sensitivity |
Specificity |
FIB-4 score |
Age, ALT, AST, platelet count |
<1.3 |
0.82 |
0.57 |
NAFLD fibrosis score |
Age, ALT, AST, platelet count, albumin, BMI, presence of impaired fasting glucose or T2DM |
<−1.455 |
0.89 |
0.37 |
FibroScan® LSM |
Elastographic method |
<9.9 kPa |
0.83 |
0.61 |
Table 3 Sensitivity and specificity of widely available non-invasive tests for advanced fibrosis (stage 3 or higher) in NAFLD43
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; FIB-4, fibrosis-4 index; LSM, liver stiffness measurement; NAFLD, non-alcoholic fatty liver disease; T2DM, type 2 diabetes mellitus
Risk stratification and fibrosis assessment
|
Recommendation 3: For each at-risk patient identified, calculate fibrosis-4 index (FIB-4) score to determine whether the patient has minimal (FIB-4: <1.3, F0-1), indeterminate (FIB-4: 1.3–2.67), or likely advanced fibrosis (FIB-4: >2.67, ≥F3) Recommendation 4: Patients with FIB-4 <1.3 have minimal fibrosis. These patients can be managed by their PCP Recommendation 5: Patients with FIB-4 1.3–2.67 have an indeterminate fibrosis score and should have a second non-invasive test such as Fibro Sure or ELF (serology-based tests) or liver stiffness (imaging based elastography) Recommendation 6: Patients with FIB-4 >2.67 have a 97% likelihood of advanced fibrosis and should be referred to a hepatologist |
Once an at-risk patient has been identified, the patient should be stratified into low (fibrosis stage 0-1), indeterminate (fibrosis stage 2), or advanced fibrosis (fibrosis stage 3 and higher) using non-invasive tests (NITs). Several NITs have been tested and validated to detect fibrosis stage in NAFLD (Table 3).43
A recent meta-analysis (64 studies, 13,046 NAFLD patients) compared a panel of tests (BARD.BMI, AST/ALT ratio, diabetes, APRI.AST to platelet ratio index, FIB-4.fibrosis-4 index, and NFS.NAFLD fibrosis score) for diagnosing advanced fibrosis with reported area under the receiver operating characteristic curve of 0.76, 0.77, 0.84, and 0.84, respectively.44 Among the four serum-based NITs, FIB-4, and NFS performed best, with negative predictive values >90%.44 FIB-4 and NFS have strong negative predictive values and predict a better long-term outcome.45,46
Here we propose the application of FIB-4 for initial risk stratification. It is a simple, inexpensive NIT and includes variables (age, AST, ALT, and platelet count) that are typically obtained during PCP clinic visits.47,48 FIB-4 is an effective tool to rule out advanced fibrosis (Table 3) and possibly avoid unnecessary referrals to specialist care.49,50 A FIB-4 cutoff of <1.3 provides a sensitivity of 0.82 and a specificity of 0.57 for excluding advanced fibrosis and a cutoff of ≥2.67 provides a sensitivity of 0.36 and a specificity of 0.93 for detecting advanced fibrosis.43 As age can influence the accuracy of the degree of fibrosis determined by FIB-4, we propose a different cut off for minimal fibrosis in patients aged <35years or >65years (Table 3).51
Patients with an indeterminate NIT should have a second test, either serology or imaging based.48 (Figure 1). Choice of test should be determined by availability and if unclear, guidance can be obtained from a hepatologist. A step-wise approach has been outlined in primary care settings, to decrease the large influx of NAFLD referrals to specialty care and utilize limited specialty resources for those at risk of advanced disease.52
The accuracy of commonly used serology-based NITs has been described above and in Table 2. Imaging-based technologies have gained popularity, most notably FibroScan®, a non-invasive, quick, reproducible, point-of-care test, with results that can be shared immediately with the patient and providers.53,54 In addition to providing information about liver stiffness, degree of hepatic steatosis can be estimated with the controlled attenuation parameter feature.55 Vibration-controlled transient elastography (VCTE) is highly sensitive and provides a negative predictive value of 99% for ruling out stage 3–4 fibrosis (Table 3).43,56,57 Factors to consider include asking the patient to fast for 3–4 hours before examination and awareness of patient characteristics that can overestimate the degree of liver stiffness, such as body habitus (accounting for 5% failure rate).
Treatment and monitoring
Recommendation 7: All patients identified with NAFLD should be counselled on lifestyle medication around weight loss and physical activity
Recommendation 8: All patients identified with NAFLD should work with their PCP to manage medical comorbidities
Recommendation 9: At each clinic visit, patient smoking status and detailed alcohol history should be obtained
Recommendation 10: All patients with NAFLD should be vaccinated for hepatitis A and B virus, receive an annual influenza virus vaccine, and a pneumococcal vaccine every 5 years Recommendation 11: Patients with no evidence of significant fibrosis (fibrosis-4 index.FIB-4 <1.3) should work with their PCP to manage medical comorbidities Recommendation 12: Patients with evidence of significant fibrosis (FIB-4 ≥1.3) should be comanaged by their PCP and referred to a hepatologist Recommendation 13: Patients with advanced fibrosis (FIB-4 >2.67) should be considered for screening for HCC every 6 months |
Lifestyle interventions are key in the clinical management of all spectrums of NAFLD.8,22,58 Weight loss is considered the cornerstone of treatment and shown to improve steatosis, liver injury, and fibrosis.59 Weight loss goals need to be measurable, reasonable, and tailored to each individual patient.60 The approach to dietary changes should incorporate MyPlate61 and individualized modification of current diet versus adoption of unsustainable, radical diets. The intake of fructose (particularly sugar-sweetened beverages) should be strongly discouraged.58 A meta-analysis of 12 studies involving 35,705 participants found that consumption of sugar-sweetened beverages increased the relative risk of NAFLD by 39%.62 While there is no specific type of exercise to be most impactful in NAFLD,58 any form of movement or fitness should be encouraged. Dietary advice,63 fitness, and weight loss goals should be included in the treatment plan.
Other lifestyle changes that should be addressed at every visit are inquiry into smoking and counselling on smoking cessation; and a detailed history of alcohol use and methods to decrease alcohol intake. Smoking is a risk factor for the development of hepatic fibrosis and HCC.64,65 In a retrospective analysis that assessed risk factors associated with long-term outcomes in 619 patients with NAFLD, in addition to fibrosis stage, smoking was associated with a 2- to 3-fold increased risk of overall and liver-related mortality.10 Heavy alcohol use is especially detrimental to patients with NAFLD. Observational studies demonstrated an increased risk of hepatic inflammation, cirrhosis, and HCC in obese and diabetic individuals who consumed >2 alcoholic drinks per day.66–68 Any quantity of alcohol use in persons with NASH-related cirrhosis increases the risk of HCC by fourfold (hazard ratio 3.8, 95% CI 1.6–8.9, P<0.01) and the risk was not modified by volume of alcohol use or former drinking.69 We recommend complete abstinence in patients with advanced fibrosis and cirrhosis.
NAFLD is part of the metabolic syndrome spectrum and so managing metabolic comorbidities is imperative. PCPs should consider managing patients with NAFLD as part of a multidisciplinary team that includes dieticians, psychologists, community healthcare workers, and exercise physiologists.60
Metformin is not recommended for NAFLD or NASH but should be considered as first-line pharmacologic therapy for those with NAFLD and T2DM. Metformin promotes weight loss, decreases body fat, and improves hepatic insulin sensitivity.70 We recommend against diabetes medications more prone to increasing weight. In patients with T2DM and NASH, GLP-1 receptor agonists or SGLT-2is can be considered as second-line agents.71 Both agents have shown secondary benefits on weight loss, glycemic control, and improvement in NASH.72–76
Dyslipidaemia in patients with NAFLD and NASH should be treated.8 Statins can be protective against HCC and liver fibrosis and they reduce cardiovascular disease morbidity and mortality.77,78 Statins can be well tolerated and efficacious in patients with compensated NASH cirrhosis, but should be avoided in patients with decompensated cirrhosis.8 Renin-angiotensin inhibitors may have antifibrotic properties and hence can be prescribed for hypertension in NAFLD and NASH patients.79
Bariatric surgery should be considered as a treatment option in patients with NAFLD who meet criteria.8 In a systematic review and meta-analysis (including 3093 patients with NAFLD), bariatric surgery resulted in resolution of steatosis in 66% of patients and resolution of fibrosis in 40%.80,81
All patients with NAFLD need longitudinal follow up. Those identified with minimal fibrosis (FIB-4 <1.3, F0-1) can be followed by PCPs to manage metabolic comorbidities, and bi-annual blood work including blood count, liver enzymes, HbA1c, and lipids to monitor and detect metabolic diseases. PCPs should calculate hepatic and cardiovascular risks every year and refer to a hepatologist and/or cardiologist if indicated. Patients with intermediate or advanced fibrosis (FIB-4 ≥1.3 and ≥2, respectively) should also be followed by primary care in consultation with a hepatologist.
All patients with a diagnosis of chronic liver disease such as NAFLD should be vaccinated for hepatitis A and B, if not already immune. We also recommend annual influenza virus vaccination and a pneumococcal vaccination every 5years. Patients with stage 3 fibrosis or higher should be considered for screening for HCC every 6months,82 because as many as 25% of these patients can develop HCC in the absence of cirrhosis.83
Like obesity and T2DM, NAFLD has become an epidemic. While liver biopsy is the mainstay for diagnosing NASH and advanced fibrosis, NITs are a cost-effective method of stratifying patients in low- and high-risk groups. This algorithm encourages the PCP to recognize the potential for end-organ damage and intervene in time to improve patient health and longevity. The stratification scheme encourages maximum use of the PCP’s time and skills and improves the utilization of specialists. PCPs are overburdened, so the addition of ancillary staff to assist with dietary and lifestyle changes are essential to the effective management of these patients.
NASHNET contracted Caudex, New York, NY, USA for professional medical writing support. Writing and editorial support, respectively, were provided by Simon Wigfield and Reza Sayeed of Caudex, Oxford, UK and Stephanie Wolfe of Caudex, New York, NY, USA.
All authors declare there are no conflicts of interest towards this manuscript.
The algorithm-development meeting and manuscript development, including professional medical writing and editorial support, were internally funded by NASHNET.

©2021 Dinani, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.