eISSN: 2373-6372


Case Report Volume 10 Issue 4
1Department of Gastroenterology and Hepatology, Sao Paulo State Public Server Hospital (IAMSPE), Brazil
2Department of Radiology, Sao Paulo State Public Server Hospital (IAMSPE), Brazil
Correspondence: Rogério Camargo Pinheiro Alves, Tutor of resident physician mentoring program in gastroenterology and hepatology of IAMSPE, Rua Pedro de Toledo, Sao Paulo, Sao Paulo, Brazil, Tel rcpalves@uol.com.br
Received: July 12, 2019 | Published: August 1, 2019
Citation: Luz LO, Costa TDFA, Alves RCP, et al. Acute pulmonary embolism as the first manifestation of hepatocellular carcinoma. Gastroenterol Hepatol Open Access. 2019;10(4):221-224. DOI: 10.15406/ghoa.2019.10.00385
We report a case of a 56-year-old man admitted to the Emergency Department of a Hospital in Sao Paulo with dyspnea, hypoxemia and pleural effusion due to pulmonary embolism as the first presenting sign of hepatocellular carcinoma. CT angiography of the chest confirmed bilateral pulmonary embolism with tumor thrombus invasion of the inferior vena cava and right atrium. The patient was not aware of previous chronic liver disease. Anticoagulant therapy with low-molecular weight heparin was administered. Due to a high risk of complications in a cardiovascular surgery intervention, the medical team decided to start palliative treatment for the HCC with sorafenib. Patient was discharged after 5days of anticoagulation without any respiratory complaint, using enoxaparin (1mg/kg, q12h). Patient was readmitted to hospital 6months after the diagnosis of HCC with acute upper gastrointestinal hemorrhage and died due to its complications. Liver cancer is the sixth most prevalent cancer, and patients with cirrhosis are at highest risk for developing hepatocellular carcinoma. Thus, these patients should be screened with abdominal ultrasound every 6months, aiming a diagnosis in early stages. A therapeutic option for a thrombus that invades inferior vena cava and the right atrium is surgery, but most patients would not tolerate such procedure due to advanced hepatic dysfunction. In a palliative scenario, sorafenib is a first line treatment option.
Keywords: hepatocellular carcinoma, pulmonary embolism, venae cavae, heart atria, case report
HCC, hepatocellular carcinoma; BCLC, barcelona clinic liver cancer; PE, pulmonary thromboembolism; IVC, inferior vena cava; RA, right atrium; IAMSPE, sao paulo state public server hospital; CT, computed tomography; PS, performance status
Liver cancer is the fifth most prevalent cancer worldwide and it is the second most frequent cause of cancer-related death.1,2,3 In 11 countries, it was the most diagnosed type of cancer for men in 2015.2 Hepatocellular carcinoma (HCC) accounts for most liver cancer deaths and treating properly hepatitis B and C would lead to a reduction in mortality, as these are the leading causes of chronic liver disease.1,2,4 HCC is characterized by a large heterogeneous distribution, and patients with chronic liver disease and cirrhosis are at highest risk of developing this malignancy.1,4 As the main risk factor for HCC is cirrhosis, patients with this disease should be screened with abdominal ultrasound every 6months, aiming a diagnosis in early stages, when the tumor is most likely to be curable by resection, ablation or even liver transplantation.1,2,4
Staging the tumor and defining the best therapeutic is based on Barcelona Clinic Liver Cancer (BCLC) staging classification, which takes into consideration patient’s performance status (PS), tumor volume, number of lesions, vascular invasion, extrahepatic spread and the degree of hepatic dysfunction.3 Advanced stage (or stage C) is characterized by the presence of portal invasion, extrahepatic spread, PS 1-2 and Child-Pugh A-B. Sorafenib is a first line of treatment in a palliative scenario.1,3,4
A tumor thrombus formation in either portal or hepatic vein is a marker of an advanced stage.5,6 Pulmonary thromboembolism (PE) as the first manifestation of HCC is rare, although vascular invasion is not. HCC is often diagnosed in advanced stages of the disease, when it is possible to detect vascular invasion and metastasis. When a thrombus invades inferior vena cava (IVC) and the right atrium (RA), it can lead to cardiac failure or pulmonary embolization.6 Thus, these patients usually have low life expectancy, with a high degree of pulmonary, cardiac and hepatic involvement, which, untimely, limit surgery.5-7
We report a case of a cirrhotic patient with HCC with tumor thrombi invasion of the IVC and RA, and bilateral PE.
A 56-year-old man was admitted to the Emergency Department of a public Hospital in Sao Paulo in September 2016 for acute dyspnea and pleuritic chest pain over the previous 6days. He referred occasional intake of alcohol in the weekends and he smoked for over 20years, but had stopped 5years ago. He denied promiscuous sex activity or drug abuse and had no knowledge of pre-existing liver disease.
At admission to hospital in September 2016, physical examination revealed a respiratory rate of 20 breaths/min, a heart rate of 105 beats/min, hypoxemia (SpO2 88%), sparse telangiectasias on the chest and mild ascites with no abdominal pain associated. Initial laboratory tests worth mentioning are displayed in Table 1. The chest radiograph and a 12-lead ECG were normal.
Test |
Values |
Normal Range |
AST |
98U/L |
8-48U/L |
ALT |
45U/L |
7-55U/L |
Amylase |
41IU/L |
40-140IU/L |
Total bilirubin |
2.16mg/dL |
0.2-1.2mg/dL |
Direct bilirubin |
0.9mg/dL |
0.1-0.4mg/dL |
Creatinine |
0.8mg/dL |
0.84-1.21mg/dL |
LDH |
277IU/L |
105-333IU/L |
Alkalin phosphatase |
416U/L |
44-147U/L |
Gamma glutamyl transferase |
396U/L |
9-48IU/L |
Potassium |
4.4mEq/L |
3.5-5.0mEq/L |
Sodium |
135mEq/L |
135-145mEq/L |
Hs-CRP |
20.14mg/L |
< 3mg/L |
Urea |
30mg/dL |
20-40mg/dL |
Hb |
13g/dL |
13.5-17.5g/dL |
Ht |
40.3% |
42-54 % |
WBC |
12,800 |
4,000-11,000 cells/cubic millimeter of blood |
Platelets |
192,000 |
150,000-400,000 platelets/mcL |
Table 1 Blood chemistry values worthy of note
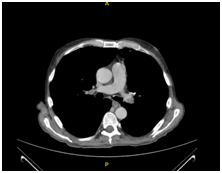
A CT angiography of the chest was performed, and a bilateral PE was found (Figure 1A). Anticoagulant therapy with low-molecular weight heparin was administered (1mg/kg, q12h). The CT angiography also revealed a liver mass and a CT of the abdomen was performed.
A B
C D
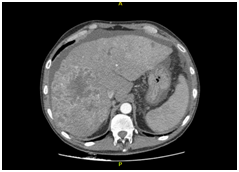
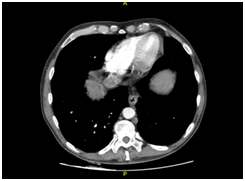
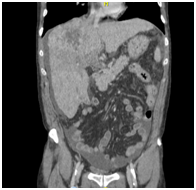
Figure 1 CT scanning. (A) Chest enhanced CT showing PE (arrow). (B) Enhanced CT of the abdominal region showing a large low-density lesion, sized 11.5x9.7 cm, in the right lobe (arrow). (C) Enhanced CT of the abdominal region showing tumour thrombus invasion of the IVC and RA (arrow). (D) Coronal reconstruction showing tumour thrombus invasion of the IVC and RA (arrow).
The CT revealed parenchymal heterogeneity of the liver, suggesting cirrhosis, and a sizeable lesion (11.5 x 9.7 cm) in the right lobe (Figure 1B). The lesion showed typical HCC image pattern, with an arterial phase enhancement and a venous/delayed phase washout. A hyperattenuating material in the portal vein (PV) lumen was also found, with no enhancement after contrast injection, inferring thrombotic/tumoral process; and a hyperattenuation in the IVC was also described (Figure 1C&D). Transthoracic echocardiogram confirmed tumoral invasion of the vena cava and right atrium.
Tumor markers results were: alpha-fetoprotein, 1,596ng/dL normal, 0-9 ng/dL; CA15-3: 27.3U/mL normal, <30; CA 125: 609.4 normal, 0-35kU/L; CA 19-9: 75.7 normal, < 37U/mL. Serologies for hepatitis B and C were negatives.
After 5days using enoxaparin, the patient had no chest pain or tachypnea. He was then discharged from the hospital, and palliative treatment for HCC with Sorafenib, 800mg/day orally was initiated. During follow-up, no side effects due to Sorafenib use were reported. Patient was readmitted to hospital 6months after the diagnosis of HCC with acute upper gastrointestinal hemorrhage and died due to its complications.
We presented in this paper a male patient with liver cirrhosis admitted to a medical service for PE as first manifestation of HCC. The hypercoagulable state of malignancy is well described in the literature. Of all cancer patients, 15% will develop symptoms related to venous thromboembolism (VTE) each year.8,9 Thus, for patients with unprovoked VTE it is reasonable to screen for occult cancer. Although metastatic dissemination of HCC is common, association with PE as a first sign of the cancer is an exceptional phenomenon in HCC cases.5,7,10
Curative treatment strategies for HCC include hepatic resection, liver transplantation and, under certain circumstances, ablative regimens such as radiofrequency or microwave ablation. In a palliative care scenario, treatment can require either locoregional and systemic strategies, or even a combination of different modalities.1,3 Some authors believe that surgery for tumor removal from the IVC and RA is the only effective treatment, but with a high surgical risk, commonly requiring cardiopulmonary bypass, fact that discourage several surgeons from performing surgery in patients with IVC and RA invasion.5,10
Tumor vascular invasion of the portal and hepatic vein is relatively common in patients with HCC, and those who develop this type of complication have a poor prognosis, and it is even worse if the tumor invades the IVC and RA, since it often leads to sudden death due to cardiac failure or pulmonary embolization.5,6,11,12 15->12 12- 13/13-> 14/14->15
Life expectancy among patients with HCC and tumor vascular invasion usually ranges from 1 to 5months without surgery, and the longest reported survival time in a case of tumor invasion with cardiac extension was 26 months.13,14 In the case presented here, the patient lived for 6months without surgery, a month longer than expected, only using systemic chemotherapy and anticoagulation.
The decision regarding treatment should always aim to increase the survival rate of patients in accordance with the concept of the best personalized therapy. In BCLC stage C (vascular invasion, extrahepatic spread, PS 1-2, Child-Pugh A-B), as in the case of our patient, when locoregional treatment options are exhausted or extrahepatic tumor dissemination is present, a systemic antiproliferative therapy is indicated.1,3
For patients who develop tumor invasion of the IVC/RA, surgery with cardiopulmonary bypass might be an option to increase life-expectancy, although patients at this stage might not tolerate such surgery due to advanced loss of liver function.5 Recently, Wei Li and colleagues reported a case of a 73year-old man with tumor invasion with cardiac extension treated with percutaneous microwave ablation (MWA), a minimally invasive percutaneous ablation of tumor thrombus, combined with tumor ablation as well. The patient was followed up for 16months, and he was still alive when they reported the case.
Our patient had decompensated cirrhosis (CHILD B), tumor vascular invasion with PE as its complication, not being eligible for a major surgery with cardiopulmonary bypass, to our conclusion. An MWA might have been a good treatment option in this scenario, but we did not have a medical team with this expertise at the time.
Currently, in cases of advanced HCC, the recommended anti-angiogenic substance is sorafenib, which reduces tumor cell proliferation and angiogenesis and it increases apoptosis by targeting tyrosine kinase receptors.1,4,15 The patient received Sorafenib for 6months, with no record of limiting side effects, and died from a complication expected in cirrhotic patients.
In cirrhotic patients, the prevalence of HCC is high, and it is mandatory to screen these patients every 6months with an abdominal ultrasound. Once the tumor is metastatic, the only treatment available is systemic chemotherapy, and nowadays there are two options available as first line treatments: sorafenib and lenvatinib.3 All cirrhotic patients with the diagnosis of PE should have a thorough image investigation of the abdomen performed to rule out HCC.
None.
We have no conflicts of interest.

©2019 Luz, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.