eISSN: 2373-6372


Review Article Volume 10 Issue 2
1Departamento de Radiología y Medicina Física, Spain
2Facultad de Medicina, Universidad de Granada, Spain
3Servicio de Radiodiagnóstico, Hospital Universitario San Cecilio de Granada, Spain
4Servicio de Nefrología del Hospital Universitario Virgen de las Nieves de Granada, Spain
Correspondence: Juan de Dios López-González Garrido, Profesor Titular de Universidad. Departamento de Radiología y Medicina Física de la Facultad de Medicina. Avda. de la Investigación 11, CP. 18016 Granada. España, Spain, Tel +34 958244246
Received: December 31, 2018 | Published: March 6, 2019
Citation: Garófano-Jerez JM, Benedicto-Hernández E, Gila JDLG, et al. Imaging evaluation of the heterotaxy syndrome. Gastroenterol Hepatol Open Access. 2019;10(2):75-80. DOI: 10.15406/ghoa.2019.10.00359
Situs ambiguus, or heterotaxy, is a rare syndrome characterized by an abnormal arrangement of the internal organs in the chest and abdomen and is usually associated with congenital heart diseases. Despite its variable presentation, heterotaxy syndrome can be classified into heterotaxy with polysplenia or left isomerism, and heterotaxy with asplenia or right isomerism. The different imaging techniques reveal the characteristic imaging findings that allow an accurate description of each patient's specific anatomy. Evaluation of the position of the following structures relative to midline is crucial: atria, cardiac apex, venous drainage, aorta, stomach, liver and gallbladder, as well as the presence or absence of intestinal malrotation, number of spleens, and presence of tri- or bilobed lungs.
We present a case report of a 42-year-old man with dextrocardia underwent laparoscopic cholecystectomy for cholelithiasis. Subsequently, a complete resection of the common bile duct required reconstruction of the biliary tract. During six years the patient underwent frequent examinations for recurrent episodes of cholangitis that required stenosis dilation and biliary stent placement using endoscopic retrograde cholangiopancreatography. Despite these treatments, the patient required surgery again for biliary stenosis, this time a bilioenteric anastomosis (Roux-en-Y) was performed. During the surgical procedure migration of the biliary stent occurred.
Keywords: embryology, genetics, left-right asymmetry, heterotaxy syndrome, situs ambiguus, imaging techniques
Heterotaxy, or situs ambiguus, is a rare syndrome-occurring in only one out of every 10,000 to 20,000 births-characterized by an abnormal organ arrangement in the chest or abdomen and is usually associated with congenital heart disease (CHD). Despite its variable presentation, there are two types of heterotaxy syndrome: heterotaxy with polysplenia or left isomerism, and heterotaxy with asplenia or right isomerism. In 50-90% of cases heterotaxy with polysplenia is associated with CHD and only 5-10% of patients will survive to adulthood.
Organ arrangement in humans begins the 3rd week of embryological development, concerned with the of gastrulation process by which the bilaminar embryo acquires a third layer to become a trilaminar disc1 (ectoderm, mesoderm and endoderm) and with the development of the craniocaudal, dorsoventral and left-right axes of the embryo.2
The establishment of the left-right axis is essential for the usual asymmetric arrangement of organs and vessels in the chest and abdomen: cardiac apex, bilobed lung, spleen, stomach and aorta on the left relative to midline; and liver, trilobed lung and inferior vena cava (IVC) on the right. Five stages of this process have been described:3,4
The initial symmetry breaking is related to the Hensen’s (primitive) node,5,6 a cluster of cells that corresponds to the cranial end of the primitive streak, that becomes a transient “embryonic organizer”.
A signal, as yet unknown, alters the nodal cells to produce two non-equivalent right and left halves. The expression of many genes differs on the left and on the right side, assigning the left and right identities. Consequently, signaling cascades are activated with the formation of large domains of asymmetric gene expression in the lateral mesoderm, which results in the normal asymmetric morphology of organs.5
Work in mouse, and by extension in mammals, has proposed the “nodal flow” model.5,6 Cells in the node have a single motile cilium and these cilia generate with a rotational motion a leftward flow of the perinodal fluid that transports morphogenic proteins.3,4,5,7 This motion would explain the asymmetric transfer of certain genes. The combination of situs abnormalities and primary ciliary dysfunction in human syndromes would strongly support this model.5
An alternative theory suggests that increased calcium levels in endodermal cells to the left of the node play an important role in the asymmetric development of organs.7,8
The genetic mechanisms involved in the control of laterality are complex and not completely understood. More than one hundred genes have been identified that play an important role in left-right pattering in animals, but only a few seem to be implicated in heterotaxy syndrome in humans.7 Most cases of heterotaxy are sporadic3 but familial cases have also been identified, with the autosomal recessive pattern of inheritance being the most common, like in primary ciliary dyskinesia. The autosomal dominant and X-linked patterns of inheritance are less common.3 Despite this, careful genetic studies support a multifactorial inheritance.9 Mutations with incomplete penetrance and variable expression suggest the influence of environmental factors such as pregestational diabetes, retinoic acid exposure and maternal cocaine use.3
The term situs refers to the ‘site or location’ of the heart, viscera and great vessels relative to midline.
Situs Solitus
Situs solitus is the normal asymmetric arrangement of the heart and organs, with the cardiac apex, bilobed lung, spleen, stomach and aorta on the left. The liver, trilobed lung and IVC are on the right. The incidence of CHD in patients with situs solitus is only 0.6%–0.8%.9,10 Defects in laterality pattering during gastrulation result in abnormal spatial arrangement of viscera and in a wide range of cardiac malformations.5,9 Situs abnormalities are classified into situs inversus and situs ambiguus.
Situs Inversus
Situs inversus totalis (Figure 1) refers to the mirror image of situs solitus. In the general population situs inversus totalis is found in 1:10000 people2 and shows a relatively low incidence of CHD (3-5%).9–11 Of less common ocurrence is situs inversus partialis2 where the cardiac apex is on the left (levocardia) but the rest of the organs maintain their mirror image position. The incidence of CHD in patients with partial situs is much higher (> 90%).10,11
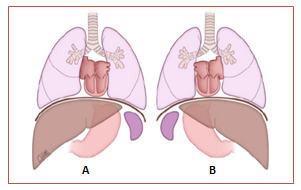
Figure 1 Drawing shows a comparison between the normal arrangement of internal organs or situs solitus (A) and its mirror image or situs inversus (B).12
Situs Ambiguus
Situs ambiguus, or heterotaxy syndrome, refers to the abnormal arrangement of organs and vessels in the thorax and abdomen relative to midline. This arrangement is different from the orderly arrangement seen in situs solitus and situs inversus.9,13 The incidence of situs ambiguus is between 1:10000 and 1:20000 births2 and is usually associated with complex heart malformations. Malformations in other organs can also be found.14 Despite the fact that a specific pattern cannot be identified in most cases, there is a tendency to bilateral visceroatrial symmetry,15 which allows differentiation in two categories (Figure 2): heterotaxy with left isomerism (polysplenia) and heterotaxy with right isomerism (asplenia).
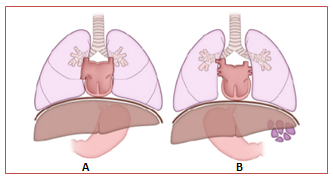
Figure 2 Drawing shows the abnormal arrangement of internal organs seen in heterotaxy syndromes. Right isomerism (A) and left isomerism (B).12
Situs Ambiguus with polysplenia (Left Isomerism)
Situs ambiguus with polysplenia, or left isomerism, is the most common form of heterotaxy and has female predominance.11,16 Left isomerism is generally characterized by centrally located abdominal organs and presence of multiple spleens;10,16 however, one single lobulated spleen or even a normal spleen is seen in some patients. The spleen or spleens are always on the same side as the stomach, typically along its greater curvature (Figure 3).
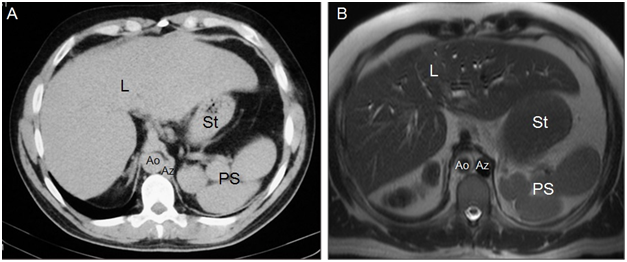
Figure 3Axial CT (A) and MR (B) images show the aorta on the right (Ao), azygos vein on the left (Az), liver is midline (L) but slightly on the right and polysplenia (PS) on the left relative to greater curvatur of the stomach (St). IVC cannot be identified. Cholecystectomy.
The most consistent anatomic finding is an interrupted suprarenal IVC11 with azygous or hemiazygous continuation, with direct hepatic drainage to the heart chambers through a common trunk. In polysplenia, both lungs are bilobed with hyparterial bronchi. Cardiac anomalies in polysplenic patients are less severe16 and less common (50-90%) than in patients with situs with asplenia. Partial anomalous venous return to the lungs, atrial septal defect, and atrioventricular canal are the most common cardiac anomalies.9 In addition, these patients have a higher risk of developing arrhythmias-as a result of the absence of the sinus node, located in the left atrium-and complete heart block.12 On the other hand, in patients with heterotaxy syndrome with polysplenia the liver and gallblader are susally located next to midline (Figures 3-5).10,14
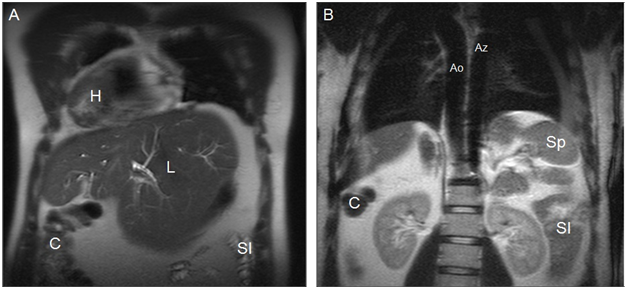
Figure 4Coronal MR images in a more anterior position (A) and in a more posterior position(B) show dextrocardia and cardiac apex on the right (H), midline liver (L) with a prominent left lobe, colon on the right (C), small intestine on the left (SI), aorta on the right (Ao), azygos vein on the left (Az), and one splenule on the left (Sp). IVC cannot be identified. Cholecystectomy.
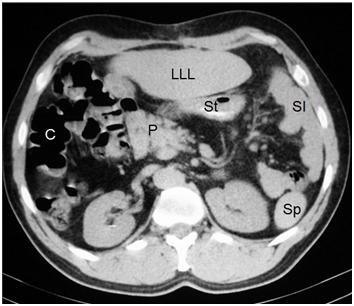
Figure 5Axial CT image shows a prominent left hepatic lobe (LLL), colon on the right (C), small intestine on the left (SI), truncated pancreas (P), left-sided stomach (St), and one splenule on the left (Sp).
In patients with biliary colics the pain is usually epigastric, therefore, a differential diagnosis should be made to rule out pancreatitis or ulcer disease.10,16 Anomalies of the biliary tract generally include biliary atresia in neonates and gallblader hypoplasia or agenesis in infancy.16 These anomalies are rarely described in adults because only 5-10% of patients survive to adolescence.16 As for the pancreas, the most common anomaly found in polysplenic patients is truncated pancreas (Figure 5).10,14,16 Intestinal malrotation is also common (70% of patients approximately) (Figures 4-6).11
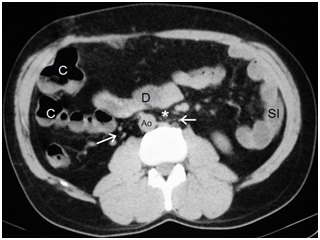
Figure 6Aorta on the right (Ao) and intestinal malrotation because the duodenum (D) does not pass between the aorta and the superior mesenteric artery (asterisk). Vascular structures that may correspond to infrarenal IVC cannot be identified, only multiple retroperitoneal serpiginous vessels (arrows), presumably venous, are identified close to the aorta.
Situs Ambiguus with asplenia (Right Isomerism)
Situs ambiguus with asplenia, or right isomerism, has male predominance and patients often present with cyanosis and severe respiratory distress.9 Unlike left isomerism, the spleen is absent, IVC and aorta are in ipsilateral position, and asplenic patients have trilobed lungs with eparterial bronchi.9
The incidence of CHD in this syndrome is close to 100% and include common atrioventricular canal, univentricular heart, transposition of the great arteries, and anomalous venous connections, most commoly anomalous pulmonary venous return.9 The complex cardiac malformations and abnormal immune status-related to spleen absence-explain the poor prognosis of this group of patients. As mentioned above regarding intestinal malrotation in left isomerism, this finding is also common in right isomerism (70% of patients approximately).11
An appropriate description of the patient’s anatomy using imaging studies is required for the diagnosis of situs inversus and heterotaxy syndrome. 9 It is critical to evaluate the position of the following structures relative to midline: atria, cardiac apex, venous drainage, aorta, stomach, liver and gallbladder, as well as the presence or absence of intestinal malrotation, number of spleens, and presence of tri- or bilobed lungs.
Generally, neonates with cyanosis or evidence of heart failure undergo an “initial test”12 that includes pulse oximetry, electrocardiogram, and chest radiograph.
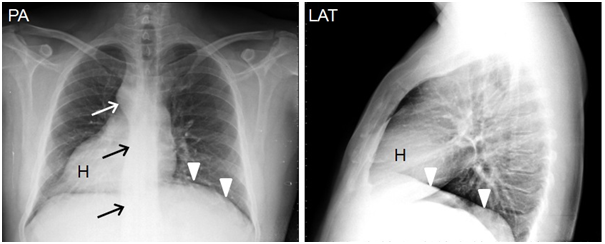
Figure 7Chest radiographs in posteranterior (PA) and lateral (LAT) projections show dextrocardia with cardiac apex on the right (H), aortic knob and descending aorta on the right (arrows), left diaphragm is in a superior position (arrowhead). Minor fissure cannot not be identified.
The diagnosis of isomerism is typically made by echocardiography, both prenatal and postnatal, which provides details of intracardiac anatomy and cardiovascular connections. In right isomerism, the aorta and IVC run adjacent and parallel to each other, with the aorta posterior to either the right or the left of the spine. In left isomerism, the aorta is often midline, an interrupted IVC with azygos continuation courses posterior to the aorta.
Once the diagnosis is stablished or in doubtful cases, other imaging techniques can be used9,12 abdominal ultrasound or CT-in order to obtain better anatomic details and evaluate the location, number and function of the spleen. MR imaging and cardioangiography are usually performed to establish the surgical planning in patients with CHD (Table 1).9
Differential diagnosis |
X-Ray findings |
Abdominal US findings |
CT findings |
Situs inversus totalis |
Concordant location of cardiac situs (dextrocardia) relative to mirror image of abdominal organs |
Stomach on right side |
Thoracoabdominal organs in mirror image compared to situs solitus |
Spleen on right side along the greater curvature of stomach |
No changes in venous drainage |
||
Liver and gallblader on left side |
|||
Situs ambiguus with polysplenia |
Heart in left hemithorax with apex to the left or to the right |
Stomach on right |
Intestine mal- or non-rotated |
Stomach bubble on right side |
One or more spleens typically located along the greater curvature of the stomach |
Bilateral bilobed lungs with hyparterial bronchi |
|
Liver often in midline |
IVC interruption with azygos or hemiazygos continuation |
||
Situs ambiguus with asplenia |
Heart in left hemithorax with apex to the left or to the right |
Stomach on right |
Intestine mal- or non-rotated |
Stomach bubble on right side |
Characteristic absence of spleen |
Bilateral trilobed lungs with eparterial bronchi |
|
Liver often in midline |
IVC ipsilateral to aorta |
||
IVC: Inferior Vena Cava |
Table 1 Imaging techniques used for the differential diagnosis of situs inversus totalis and situs ambiguus.11
Situs inversus is usually an incidental finding. These patients seek medical attention for common conditions in the clinical pratice that require diagnostic imaging studies, which reveal the abnormal left-right arrangement of the thoracoabdominal organs.2
Establishing the diagnosis and classification of heterotaxy syndromes may be difficult due to their great variability, both anatomic and clinical.14 None of the findings is pathognomonic.
Most cases of right isomerism are identified during childhood due to the presence of severe cyanotic heart diseases,14 as well as asplenia, which results in a higher risk of severe and fatal infections.17,18 However, the diagnosis of left isomerism is more difficult due to the wide range of heart defects associated to this condition, including mild forms that do not require surgery, and the absence of autoimmune disorders. The situs may go unnoticed until adulthood, when these patients undergo imaging procedures for other reasons like cholecystitis or appendicitis.
Management of patients with heterotaxy syndrome is complex and dictated by the nature and severity of the anatomic heart defects and extracardiac abnormalities.
Treatment for heart deffects include:19
In addition, the treatment of extracardiac abnormalities includes:19
The prognosis of both left and right isomerism is directly related to the associated anomalies, congenital or acquired, seen in these syndromes.2 Most patients with situs inversus can lead a healthy life, but cardiac anomalies and Kartagener syndrome are present in approximately 1 out of 20 and 1 out of 5 patients, respectively.2 Prognosis in heterotaxy syndromes highly depends on the severity of the cardiac malformations.16 Patients with complex cardiac malformations have a poor prognosis. Mortality of asplenic patients during the first year of life is over 85%, and over 50% for polysplenic patients.8,11 Only 5-10% of polysplenic patients without cardiac disease survive to adulthood.21
Wang et al.22 have described the possibility of prenatal diagnosis of left and right isomerism using the correlation to some ultrasound findings. These authors make a classification based on the position of the heart that could really benefit prenatal management and follow up, therefore improving the prognosis.
Heterotaxy syndrome is rarely seen in adults but can be diagnosed incidentally. For this reason, understanding the visceral abnormalities present in this syndrome in order to avoid is important to rule out other pathologic conditions. Despite the wide variety of anatomic anomalies that can be seen in heterotaxy, a classification can be made based on isomerism: heterotaxy with left isomerism (polysplenia), and heterotaxy with right isomerism (asplenia).
The use of echocardiography and other imaging techniques is essential for diagnosis as they allow an adequated description of the arrangement of thoracoabdominal organs. Heterotaxy syndrome management includes the medical and surgical treatment of the possibly associated CHD as well as the treatment of anomalies in other organs such as asplenia, intestinal malrotation and biliary atresia. The prognosis is dictated by the severity of cardiac malformations; during the first year of life the mortality of patients with right isomerism is over 85%, and over 50% for patients with lef isomerism. Only 5 to 10% of patients with left isomerism and no cardiac malformations survive to adulthood.
We present a case report of a 42-year-old man with situs ambiguus with polysplenia. Unfortunately, it was not possible to establish the time of diagnosis and wheter this case was sporadic or familial. As we have been discussing along this work, the diagnosis of heterotaxy syndrome may be complex due to its variable presentation and although some findings are characteristic, none of them is pathognomonic.
In the particular case of heterotaxy with polysplenia, the diagnosis is more difficult and can be delayed because of its association with a wide range of CHD9,12 that go from the most severe forms, with symptoms appearing at birth (cyanosis, heart failure) and requiring surgerical procedures, to mild forms that may go unnoticed.
Additionally, differential diagnosis–mainly based on echocardiography-with other complex CHD12 and with situs ambiguus with asplenia is required as patient management is highly variable.
Cholangiography MR and CT images confirm the diagnosis of ‘situs ambiguus with polysplenia’. Both imaging techniques reveal the abnormal arrangement and anatomy of the thoracoabdominal organs characteristic of heterotaxy in this patient:
Dextrocardia with cardiac apex to the right (Figures 4) (Figure 8).
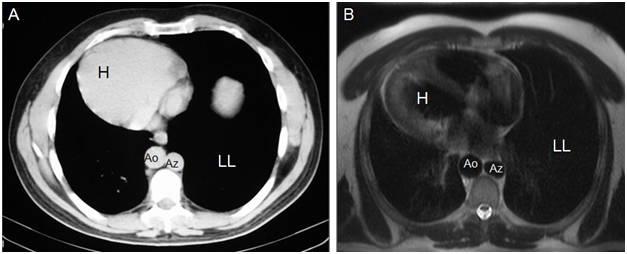
Figure 8Axial CT (A) and MR (B) images show dextrocardia and cardiac apex on the right (H), aorta on the right (Ao), azygos vein on the left (Az), and a larger left lung (LL).
Follow-up chest radiographs (Figure 7) after the previously mentioned imaging produces shows dextrocardia with cardiac apex to the right; right hilum slighty superior to the left hilum; the aortic knob is observed over the right bronchus and the thoracic descending aorta is on the right; left diaphragm in a higher position and below it the gastric bubble is observed in some follow-up images. Nonetheless, the minor fissure cannot be identified in any of the follow-up radiographs and therefore the presence of a trilobed lung cannot be confirmed, although other findings are suggestive of a trilobed left lung. As for the management and treatment of this patient, taking into consideration that he has reached the age of 42 years and very few patients with situs ambiguus survive into adulthood–only 5-10%21 of polysplenic patients without heart disease-, we can come to the conclusion that the presence of heterotaxy does not seem to have caused severe health problems throughout his life that had required medical or surgical treatment specific to this syndrome. For the same reasons, we can assume that this patient’s prognosis is ‘good’.
None.
Authors declare that there is no conflict of interest.

©2019 Garófano-Jerez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 IBS awareness month is April, which is very common syndrome globally, so to develop awareness about the irritable bowel syndrome (IBS), we are eager to publish articles to spread acquaintance to all our readers. So, take the opportunity in sending your valuable articles to raise alertness to the global people and also get the best discount of 30% for your submissions to our Gastroenterology & Hepatology: Open access (GHOA).
IBS awareness month is April, which is very common syndrome globally, so to develop awareness about the irritable bowel syndrome (IBS), we are eager to publish articles to spread acquaintance to all our readers. So, take the opportunity in sending your valuable articles to raise alertness to the global people and also get the best discount of 30% for your submissions to our Gastroenterology & Hepatology: Open access (GHOA).