eISSN: 2373-6372


Aim: The aim of this study was to determine the prevalence of acute liponecrotic (aLnP), acute relapsing liponecrotic (aRelLnP), and chronic liponecrotic pancreatitis (chrLnP) in RA, and analyze the possible role of systemic vasculitis of autoimmune origin (A-SV) in the pathogenesis of liponecrotic pancreatitis (LnP), furthermore to assess the predictive clinical laboratory parameters for LnP and A-SV.
Patients and methods: At the National Institute of Rheumatology 9475 patients died between 1969 and 1992; among them 161 with RA and all of them were autopsied. RA was confirmed clinically according to the criteria of the American College of Rheumatology (ACR). Tissue samples of pancreas were available in 118 of 161 patients. Prevalence and histological patterns of pancreatitis were determined at autopsy and characterized histologically. Demographics and laboratory parameters of different patient cohorts were compared with the Student (Welch) t-probe. The relationship between aLnP or aRelLnP and A-SV, furthermore between chrRelP and A-SV were analyzed by Pearson's chi-squared (χ2) test.
Results: Pancreatitis with multiple liponecrotic foci (LnP) were found in 15 (12.71 %) of 118 patients; aLnP existed in 8 (53.33 %), aRelLnP in 4 (26.67 %), and liponecrotic foci in combination with chronic fibrotic pancreatitis (chrLnP) in 3 (20.0 %) of these 15 patients. A-SV complicated RA in 25 (21.18%) of 118 patients, and the pancreatic blood vessels were involved in 9 (36.00%) of these 25 patients; in 16 (64.00%) of 25 patients vasculitis was not found in the pancreas. The RA started later in female patients, complicated or associated with LnP, (55.4 years versus 50.15 at onset of disease), and led notably earlier to death (within 7.44 years versus 14.93); the difference of latter was significant (p <0.0396). The mean age of RA female patients, complicated by A-SV was significantly higher at onset of disease (60.57 years versus 48.33; p <0.0007), and the female patients with A-SV died significantly earlier (within 10.0 years versus 15.09; p <0.045). The link between A-SV and LnP was positive and significant (association’s coefficient=0.7539, c²=8.8418, p<0.003), which was resulted by the very strong positive correlation between A-SV and aRelLnP (c²=17.6949, p<0.0000). The relationship between A-SV and aLnP (association’s coefficient negative, (c²=0.0231, p<0.88) or A-SV and chrLnP (association’s coefficient negative, c²=0.3570, p <0.55) was not significant.
Discussion and conclusion: The risk of LnP or A-SV is higher in elderly female RA patients, and their chance of survival is lower than in males, or compared with RA patients who did not have LnP or A-SV. A-SV, as a basic complication of RA, should be regarded an important vasculogenic factor in the pathogenesis of aRelLnP, which may be regarded as a special manifestation of autoimmune pancreatitis or a vasculogenic entity in RA. The clinical significance of laboratory parameters (elevated erythrocyte sedimentation rate, increased C-reactive protein level, anemia, thrombocytosis, hypoalbuminemia, and a positive rheumatoid factor) mentioned in the literature for diagnosis of A-SV were confirmed by our study and we found further differences regarding the Latex fication, BUN and creatinin levels. Unfortunatelly they are not specific for vasculitis and do not predict vasculitis. They are related to the basic activity of RA, to renal complications of RA or to the actual intensity of inflammatory processes of the disease. In cases of suspected vasculitis the biopsy remains the gold standard.
Elevated diastase values were characteristics for LnP in agreement with the literature. The levels of GGT showed significant differences between patient cohorts with and without LnP or compared to the total population of RA patients. The study is recommended to general physicians, who may meet patients with autoimmune disorders or are interested in autoimmune diseases.
Keywords: Rheumatoid arthritis, pancreatitis, systemic vasculitis of autoimmune origin, laboratory parameters
RA, rheumatoid arthritis; ARA, american college of rheumatology, TIGAR-O, (Toxic-Metabolic, Idiopathic, Genetic, Autoimmune, Recurrent and Severe Acute Pancreatitis, Obstructive) classification system; LnP, liponecrotic pancreatitis; aLnP, acute liponecrotic pancreatitis; aRelLnP, acute relapsing liponecrotic pancreatitis; chrP, chronic pancreatitis; chrRelP, chronic relapsing pancreatitis; chrLnP, chronic liponecrotis pancreatitis; A-SV, systemic vasculitis of autoimmune origin; H-E, hematoxylin-eosin staining; ESR, erythrocyte sedimentation rate; CRP – C, reactive protein; BUN, blood urea nitrogen; RBC, red blood cells; WBC, white blood cells; LDH, lactate dehydrogenase; GPT, glutamic- pyruvic-transaminase; GGT, gamma-glutamyl-transpeptidase; SD, standard deviation
The prevalence of pancreatitis is higher in rheumatoid arthritis (RA) than in the general population.1 According to the Mayo Clinic staff the most common conditions associated with pancreatitis are “alcoholism, gallstones, abdominal surgery, certain medications, cigarette smoking, cystic fibrosis, family history of pancreatitis, hypercalcemia, which may be caused by an overactive parathyroid gland (hyperparathyroidism), high triglyceride levels in the blood (hypertriglyceridemia), infection, injury to the abdomen, pancreatic cancer or endoscopic retrograde cholangiopancreatography (ERCP)”,2 confirmed by others.3–5 Sometimes the cause for pancreatitis cannot be found.2,6–8
The TIGAR-O classification system categorizes known causes and risk factors that may interact and produce acute, acute recurrent or chronic pancreatitis.9,10 Acute, acute recurrent and chronic pancreatitis represent a disease continuum.10 The progression of acute pancreatitis to a chronic one may be evident.11,12 The initial and most characteristic feature of acute liponecrotic pancreatitis (aLnP) is acinar cell necrosis followed by a more or less pronounced inflammatory reaction, and later glandular atrophy and fibrosis.13,14 Recurrent harmful effects (repeatedly acting risk factors) may result in acute relapsing liponecrotic (aRelLnP) or chronic liponecrotic pancreatitis (chrLnP), depending on the stage of progression.13,14
The aim of this study was to determine the prevalence of acute liponecrotic (aLnP), acute relapsing liponecrotic (aRelLnP), and chronic liponecrotic pancreatitis (chrLnP) in RA, and analyze the possible role of systemic vasculitis of autoimmune origin (A-SV) in the pathogenesis of liponecrotic pancreatitis (LnP), furthermore to assess the predictive clinical laboratory parameters for LnP and A-SV. Our study focused on the relationship of LnP and A-SV; risk factors for pancreatitis such as alcohol consumption, smoking, drugs, body weight, family history etc. were not evaluated.
At the National Institute of Rheumatology 9475 patients died between 1969 and 1992; among them 161 with RA and all of them were autopsied.15 RA was confirmed clinically according to the criteria of the American College of Rheumatology (ACR).16
Tissue samples of pancreas were available or sutable for histologic evaluation without autolytic demage in 118 of 161 patients. Prevalence and histological patterns of pancreatitis were determined at autopsy and characterized histologically.13,14 The presence of A-SV was confirmed by a detailed review of extensive histological material in agreement with the recommendations of the Consensus Conference (2013),17 Scott et al.,18 and Schilling and Fassbender.19 From each patient a total of 50-100 tissue blocks of 12 organs (heart, lung, liver, spleen, kidneys, pancreas, gastrointestinal tract, adrenal glands, skeletal muscle, peripheral nerve, skin and brain) were studied microscopically.
The correlations were determined by the Student (Welch) t-probe,20 comparing the age, sex of patients, onset of RA, duration of disease, and laboratory parameters (Latex, Waaler-Roose values, ESR, CRP, albumin/globulin ratio, serum electrophoresis (albumin, alpha-1-globulin, alpha-2-globulin, beta-globulin, gamma-globulin), RBC, hemoglobin, WBC, systolic and diastolic blood pressure, blood urea nitrogen (BUN), serum creatinine, serum potassium and sodium values, urine specific gravity, proteinuria, urine sediment (RBC, WBC), serum bilirubin, LDH, GPT, GGT, blood sugar, and diastase values) at the last hospitalization: with and without LnP or A-SV. The rerelationship between aLnP or aRelLnP and A-SV, furthermore between chrRelP and A-SV were analyzed by Pearson's chi-squared (χ2) test.20
Histologic patterns of pancreatitis13,14
Liponecrotic pancreatitis (LnP) –multiple acinar liponecrotic foci, with or without inflammatory reaction, with or without hemorrhages
Acute liponecrotic pancreatitis (aLnP) – acinar liponecrotic foci usually in the same stage and similar size of necrosis, with or without inflammatory reaction, with or without hemorrhages
Acute relapsing liponecrotic pancreatitis (aRelLnP) – acinar liponecrotic foci in different stage and size of necrosis, with or without inflammatory reaction, hemorrhages, calcification (saponification) or liquefaction (pseudocyst formation)
Chronic pancreatitis (chrP) – multifocal or diffuse fibrotic interstitial pancreatitis with more or less explicit (pronounced) glandular atrophy, with or without ductal changes: plugges (concentrated secretum of exocrine glands), ductal dilatation (ductectasia), ductal proliferation, and metaplasia
Chronicrelapsing pancreatitis (chrRelP) – focal accentuated diffuse fibrotic interstitial pancreatitis usually with pronounced glandular atrophy and ductal changes
Chronic liponecrotic pancreatitis (chrLnP) – liponecrotic foci in combination with histological characteristics of chrP or chrRelP
Edematous inflammatory pancreatitis or “serous” infection associated pancreatitis (eIP) – usually a mild diffuse edematous inflammatory interstitial pancreatitis without acinar cell necrosis or hemorrhages
The prevalence of chrP, chrRelP and eIP were not evaluated in this study
"Prevalence" of vasculitis – concerns the presence of inflammatory infiltration and structural changes in blood vessels of different calibers
Systemic vasculitis of autoimmune origin (A-SV) – A-SV was defined as one of the basic manifestations of RA,15 excluding other causes of vasculitis, like hypertension, diabetes mellitus, tumors, septic infections, etc.
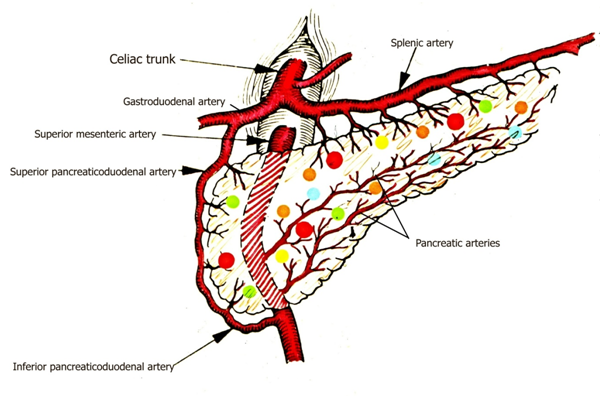
Size of blood vessels21 in tissue samples with branches of splenic artery, upper and lower gastroduodenal arteries
Arteriole (a) – no internal or external elastic membrane, <500 micrometers in diameter
Small artery (A) – only internal elastic membrane present, vessels 500-1000 micrometers in diameter
Medium size artery (AA) – internal and external elastic membrane are present – vessel >1000 micrometers in diameter
Venule (v), small vein (V), medium size vein (VV) –accompanying (a), (A) or (AA)
Multiple liponecrotic foci (LnP) were found in 15 (12.71 %) of 118 patients; aLnP existed in 8 (53.33 %), aRelLnP in 4 (26.67 %), and liponecrotic foci in combination with chronic fibrotic pancreatitis (chrLnP) in 3 (20.0 %) of these 15 patients.
A-SV complicated RA in 25 (21.18%) of 118 patients, and the pancreatic blood vessels (branches of splenic artery, upper and lower gastroduodenal arteries) were involved in 9 (36.00%) of these 25 patients & 16 (64.00%) of 25 patients vasculitis was not found in the pancreas.
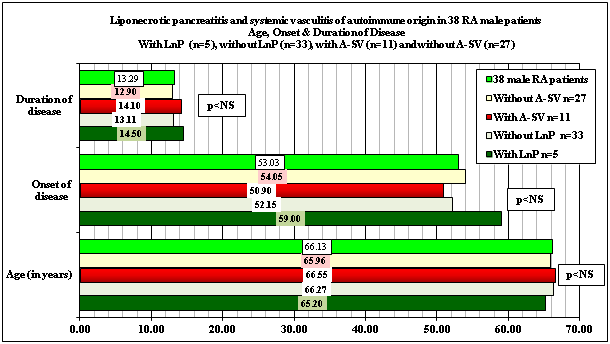
Demographics, onset and duration of disease associated with or complicated by LnP and A-SV are summarized in Table 1 and Figure 1.
Sex |
Number of autopsies |
Mean age in years at death |
Range |
Mean age at onset of disease |
Disease duration (in years) mean |
RA patients |
161 |
65.32±12.95 |
16–88 |
50.83±16.96 |
14.43±10.51 |
Female |
116 |
64.95±11.79 |
16–87 |
50.19±15.70 |
14.79±10.65 |
Male |
45 |
66.29±15.50 |
19–88 |
52.57±19.88 |
13.46±10.08 |
RA pts with pancreas |
118 of 161 |
64.97±12.84 |
16– 88 |
51.44±16.80 |
13.84±10.40 |
Female |
80 |
64.41±11.95 |
16–87 |
50.75±15.03 |
13.84±10.43 |
Male |
38 |
66.16±14.48 |
19–88 |
53.03±20.20 |
13.29±10.31 |
With LnP |
15 of 118 |
65.13±14.12 |
32–87 |
56.63±13.03 |
9.79±8.19 |
Female |
10 |
65.10±11.43 |
51–87 |
55.44±12.72 |
7.44±7.64 |
Male |
5 |
65.20±18.35 |
32–82 |
59.00±13.32 |
14.50±7.16 |
Without LnP |
103 of 118 |
64.95±12.65 |
16–88 |
50.75±17.13 |
14.38±10.54 |
Female |
70 |
64.31±12.01 |
16–84 |
50.15±15.19 |
14.93±10.43 |
Male |
33 |
66.27±13.80 |
19–88 |
52.15±20.88 |
13.11±10.68 |
With A-SV |
25 of 118 |
66.80±11.64 |
32–83 |
56.54±15.56 |
11.71±11.01 |
Female |
14 |
66.93±12.48 |
32–82 |
60.57±9.17 |
10.00±7.00 |
Male |
11 |
66.55±10.44 |
53–83 |
50.90±20.22 |
14.10±14.58 |
Wtihout A-SV |
93 of 118 |
64.48±13.11 |
16–88 |
49.87±16.86 |
14.50±10.11 |
Female |
66 |
63.88±11.76 |
16–87 |
48.33±15.21 |
15.09±10.88 |
Male |
27 |
65.96±15.83 |
19–88 |
54.05±20.11 |
12.90±7.42 |
Table 1 Sex, mean age with SD, range, onset and disease duration (in years) of RA patients with or without LnP (n=15) and A-SV(n=25) of 118 RA patients
Glossary to Table 1
RA, rheumatoid arthritis; LnP, liponecrotic pancreatitis; A-SV, systemic vasculitis of autoimmune origin
SD, standard deviation
There was no significant difference in survival time, onset or duration of RA between patient cohort’s of 161 and 118 patients (p< 0.82, p< 0.78, p< 0.67), neither between female (p< 0.76, p< 0.81, p< 0.67) nor between male (p< 0.97, p< 0.93, p< 0.95). Comparing the age, sex, onset of RA, and duration of disease at the time of death there was no significant difference in survival time, onset and duration of disease between patients with LnP and without LnP or with A-SV and without A-SV of 118 RA patients (Table 1 and Figure 1).
Demographics, onset and duration of RA in patients with LnP (n=15) and without LnP (n=103) or with A-SV (n=25) and without A-SV (n=94) of 118 RA patients (Figure 1).

RA started later in female patients, complicated by or associated with LnP, (55.4 years versus 50.15 at onset of disease), and led notably earlier to death (within 7.44 years versus 14.93); the difference of latter was significant (p<0.0396). The mean age of RA female patients, complicated by A-SV was significantly higher at onset of disease (60.57 years versus 48.33; p<0.0007), and the female patients with A-SV died significantly earlier (within 10.0 years versus 15.09; p<0.0446) (Table 1) (Table 2).
Comparing the age, sex, onset of RA, and duration of disease at the time of death there was no significant difference between female (p <0.85, p<0.33, p<0.039) and male (p<0.91, p<0.47, p<0.78) RA patients with LnP (p<0.96, p<0.19, p<0.11) and without LnP, except duration of RA of female patients;
and between female (p <0.43, p <0.0007, p <0.0446) and male (p <0.90, p<0.70, p<0.82) RA patients with A-SV (p<0.40, p<0.08, p<0.28) and without A-SV, except onset and duration of RA of female patients (Table 2)(Figures 2.1 & 2.2).
RA patients n=161 |
Age |
Onset of disease |
Disease duration |
RA pts. n=161 versus pts with pancreas n=118 |
p <0.82 |
p <0.78 |
p <0.67 |
Female n=116 versus n=80 |
p <0.76 |
p <0.82 |
p <0.67 |
Male n=45 versus n=38 |
p <0.97 |
p <0.93 |
p <0.95 |
RA pts. n=118 versus RA with LnP n=15 |
p <0.97 |
p <0.24 |
p <0.15 |
Female n=80 versus n=10 |
p <0.87 |
p <0.38 |
p <0.064 |
Male n=38 versus n=5 |
p <0.93 |
p <0.52 |
p <0.80 |
with LnP n=15 versus without LnP n=103 |
p <0.96 |
p <0.19 |
p <0.11 |
Female n=10 versus n=70 |
p <0.85 |
p <0.33 |
p <0.0396 |
Male n=5 versus n=33 |
p <0.91 |
p <0.47 |
p <0.78 |
RA pts. n=118 versus RA with A-SV n=25 |
p <0.50 |
p <0.17 |
p <0.40 |
Female n=80 versus n=14 |
p <0.51 |
p <0.004 |
p <0.09 |
Male n=38 versus n=11 |
p <0.92 |
p <0.79 |
p <0.88 |
with A-SV n=25 versus without A-SV n=93 |
p <0.40 |
p <0.08 |
p <0.28 |
Female n=14 versus n=66 |
p <0.43 |
p <0.0007 |
p <0.0446 |
Male n=11 versus n=27 |
p <0.90 |
p <0.70 |
p <0.82 |
Table 2 The statistical correlations (“p” values of significance) between female and male RA patients with and without LnP or A-SV
Glossary to Table 2
RA, rheumatoid arthritis; LnP, liponecrotic pancreatitis; A-SV, systemic vasculitis of autoimmune origin

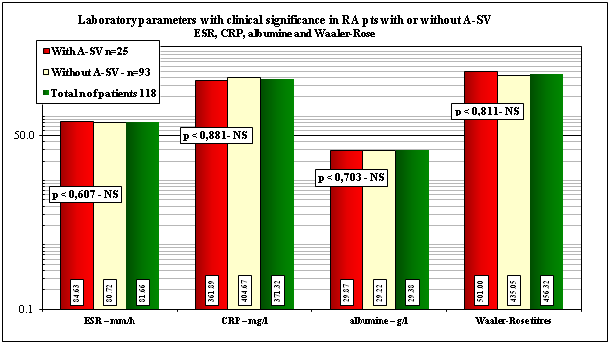
Mean values of pertinent clinical laboratory parameters with and without A-SV or in total population of RA patients at death with “p”values of correlation are shown in Table 3 and Figures 3.1 & 3.2.
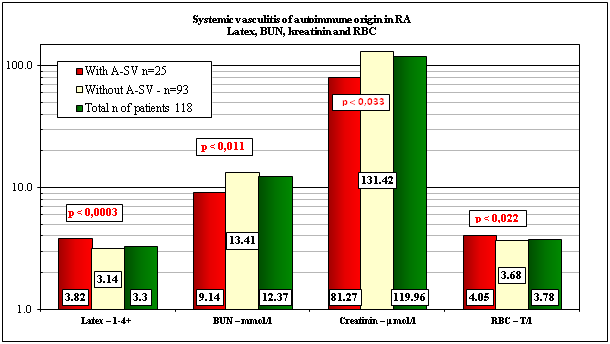
The patients with A-SV had significantly higher values of Latex fixation compared those to the patients without A-SV, and higher levels of serum carbamide, and creatinin in comparison to the normal laboratory range.
The RA patients were anemic in all groups in comparison to the normal laboratory range of RBCs (Table 3 & Figure 3.1).
Mean values of laboratory parameters±SD |
With A-SV |
Without A-SV |
Total n of pts |
Normal range |
p < 25 vs 93 and |
Latex–1-4+ |
3.82±0.49 |
3.14±1.23 |
3.30±1.14 |
0+ |
0.0003; 0.0014 |
BUN–mmol/l |
9.14±3.25 |
13.41±12.41 |
12.37±11.06 |
2.00-8.90 mmol/l |
0.011; 0.018 |
Creatinin–μmol/l |
81.27±33.02 |
131.42±102.50 |
119.96±93.809 |
62-106 μmol/l |
0.0033; 0.008 |
RBC–T/l |
4.05±0.538 |
3.68±0.711 |
3.78±0.687 |
4.50-5.90 T/l |
0.022; 0.073 |
ESR–mm/h |
84.63±29.97 |
80.72±36.3 |
81.66±34.92 |
≤15-20 mm/h |
0.607, 0.681–NS |
CRP–mg/l |
361.89±649.5 |
404.67±994.7 |
371.32±739.8 |
0.00-5.00 mg/l |
0.881, 0.907–NS |
albumine–g/l |
29.87±5.18 |
29.22±6.175 |
29.38±5.95 |
35-50 g/l |
0.703, 0.764–NS |
Waaler-Rose titre |
501.0±962.4 |
435.05±1033 |
456.32±1011 |
1:160 |
0.811, 0.862–NS |
Table 3 The mean values of clinical laboratory parameters with and without A-SV or in total population of RA patients at death and the “p” values of significance.
Glossary to Table 3 (Significantly different links are in red)
RA, rheumatoid arthritis; A-SV, systemic vasculitis of autoimmune origin; ESR, erythrocyte sedimentation rate
CRP–C, reactive protein; SD, standard deviation
The mean values of clinical-laboratory parameters with clinical significance (ESR, CRP, albumin, and rheuma facor), were elevated with and without A-SV or in total number of RA patients as well, but these differences were not significant in our patient cohorts (Table 3 & Figure 3.1).
The pertinent clinical laboratory parameters with and without LnP or in total number of RA patients at death are shown in Table 4 & Figure 4. The values of lipase values were determined occasionally at death; not mentioned in Table 4 and Figure 4.
Mean values of laboratory parameters±SD |
With LnP n=15 |
Without LnP - n=103 |
Total n of patients 118 |
Normal range |
p<vs 103 and vs. 118 RA pts |
GGT U/l |
14.20±5.15 |
69.38±64.3 |
64.08±63.28 |
11-50 U/l |
0.00000086; 0.0000014 |
Serum diastase U/l |
1842.50±2848.74 |
293.79±326.16 |
563.13±1357.88 |
28-100 U/l |
0.416–NS; |
Table 4 The clinical laboratory parameters with and without LnP or in total number of RA patients at death and and the “p” values of significance.
Glossary to Table 4
RA, rheumatoid arthritis; LnP, liponecrotic pancreatitis; GGT, gamma-glutamyl transpeptidase; SD, standard deviation
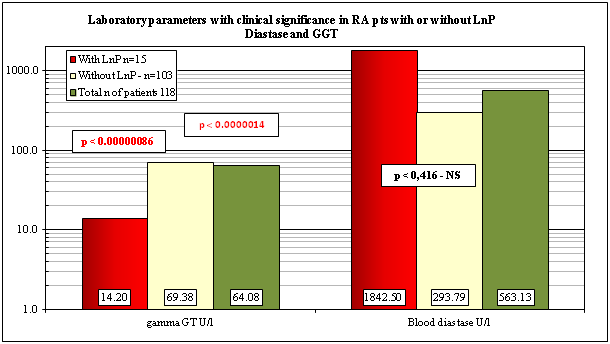
The laboratory data of patients with LnP revealed significantly lower levels of serum GGT (p < 0.00000086) in comparison without LnP or compared to the total population of RA patients (p < 0.0000014). The serum blood diastase values were higher with LnP (1842.50±2848.74) than in patients without LnP (293.79±326.16) or in total number of RA patients, but these differences were not signiificant (Table 4).
A-SV was associated with aRelLnP in 3 (20.0 %) of 15 cases; A-SV was not associated with aLnP or chrLnP. The link between A-SV and LnP was positive and significant (association’s coefficient=0.7539, c²=8.8418, p <0.003), which was resulted by the very strong positive correlation between A-SV and aRelLnP (association’s coefficient=0.9736, c²=17.6948, p<0.0000). The relationship between A-SV and aLnP (association’s coefficient negative: -1, c²=0.0231, p<0.88) or A-SV and chrLnP (association’s coefficient negative: -1, c²=0.3570, p<0.55) was not significant.
The aLnP and aRelLnP are demonstrated in Figures 5 & Figure 6. The acute-subacute-cubchronic-chronic stages of non-specific A-SV (Figures 7-9), in combination with fibrinoid necrosis (Figure 10), with acinar liponecrotic foci are demonstrated in Figures 10-11.
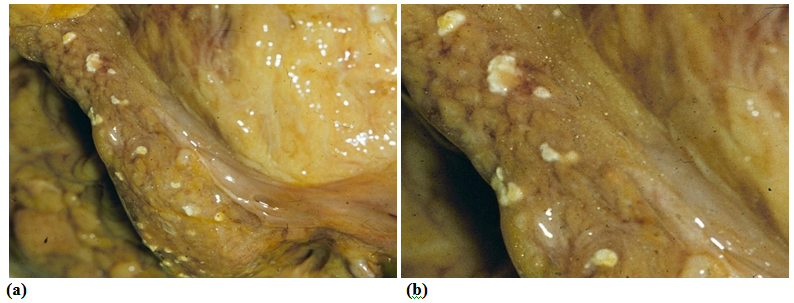
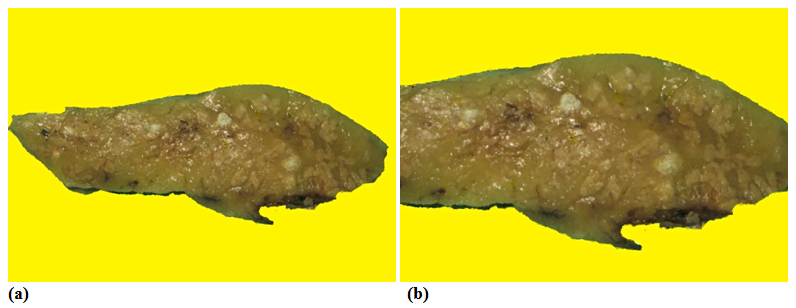
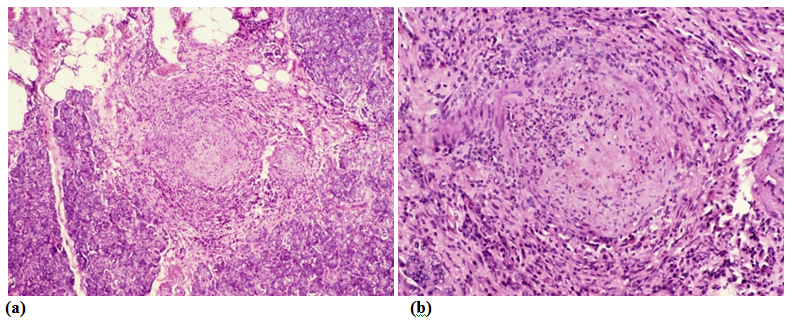
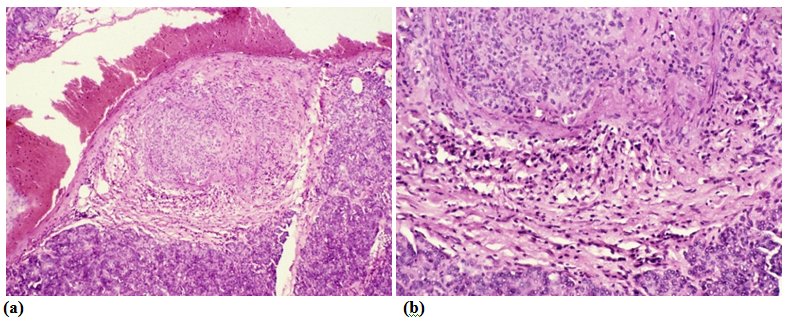
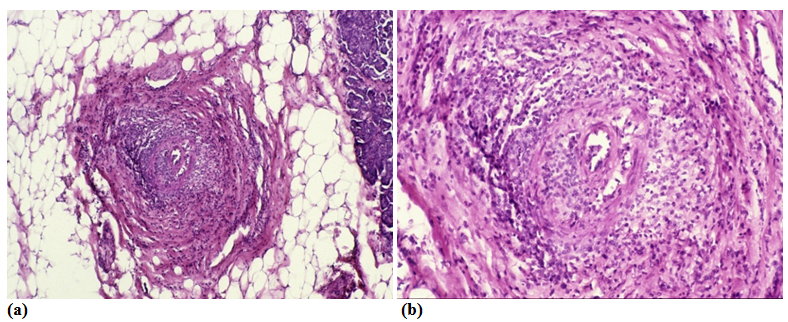

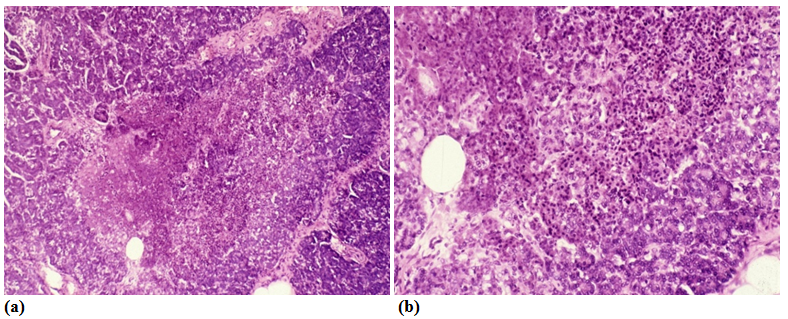
(Original magnifications correspond to the 24x36 mm transparency slide – the correct height: width ratio is 2:3. The printed size may be different, therefore it is necessary to indicate the original magnifications corresponding to a fixed size.)
The most common causes of aLnP or aRelLnP are gallstones. Trauma to the abdomen, lipid (triglyceride) disorders (hyperlipoproteinemia), hyperparathyroidism, reflux of duodenal content, and acute alcohol abuse may also have a role of in the pathogrenesis of acute pancreatitis.7,14 Certain medications (such as immunosuppressants, estrogens, thiazide diuretics, and azathioprine) can cause acute pancreatitis as well.7 The role of a vascular factor, e.g. blockage of blood vessels cannot be excluded as an etiologic factor of LnP as well.14 The cause of acute pancreatitis is unknown in 10 to 15% of patients and is considered idiopathic.7,8
The most important causal factor of chrP or chrRelP is chronic alcoholism7,8,13,14 but occasionally hyperparathyroidism may also be associated with chrP or chrRelP.2,14 The cause of chronic pancreatitis cannot be identified in about 25 -30% of patients.7,8,10,11
Chang et al.1 concluded in their cohort study, that the patients with RA are at higher risk for acute pancreatitis compared to those without RA.1 In our study the risk of LnP or A-SV was higher in elderly female RA patients at later onst of RA (55.4 years versus 50.15, and 60.57 years versus 48.33 resp.). Female RA patients with LnP or A-SV died earlier (within 7.44 years versus 14.93, and 10.00 years versus 15.09 resp.) in comparison with males or with female RA patients without LnP or A-SV.
A-SV, as a basic complication of RA, should be regarded an important pathogenetic factor of LnP,22–26 which was responsible for a fifth of 15 LnP in our study. A-SV associated exclusively with aRelLnP only in 3 (75.0 %) of 4 patients, and AAa was associated with one (25.0 %). There was a very stong relationship between A-SV and aRelLnP (c²=17.6949, p<0.0000). In these 4 (26.66 %) of 15 LnP patients aRelLnP was considered as consequence of A-SV or AAa, and therefore were regarded as an indirect complication of RA; the rest 11 (73.33 %) of 15 LnP were considered an associated disease in RA.
Vasculitis of the pancreatic arterioles and small arteries (branches of splenic artery, upper and lower gastroduodenal arteries) can lead to local ischemia and to regressive changes in the pancreatic gland. This process may be more or less widespread and multifocal, depending on the number of involved vessels. The size of necrobiotic areas is determined by the size of involved blood vessels. This vasculogenic multi- (micro-) focal necrosis of the pancreas is followed by reactive inflammation and later by fibrosis etc., depending on the stages of the pathological process. Hemorrhages, calcification (saponification) or liquefaction (pseudocyst formation) can diversify the process. Because of the recurrent nature of autoimmune vasculitis the regressive changes accumulate within the pancreas with time, and exist in different stages at death. Different sizes and stages of focal necrosis, and the co-existent vasculitis, furthermore the lack of ductal or acinar abnormalities supports the histological diagnosis of vasculogenic pancreatitis (vasculogenic origin of aRelLnP). The progressive and relapsing process in the pancreas may cause recurrent abdominal symptoms. Spontaneous remissions insidiously may lead to metabolic irregularities resistant to therapy.
The anatomic backgroung of aRelLnP caused by relapsing A-SV is presented schematically in Figure 12. Based on the strong and significant association between aRelLnP and A-SV, this form of pancreatitis may be regarded as a special manifestation of autoimmune vasculitis (a new vasculogenic entity in RA) or may be considered as a special vasculogenic type of autoimmune pancreatitis not mentioned in compilation of Mayo clinic staffs.27 Plausibly similar vasculogenic changes of the pancreas may be expected in other autoimmune diseases (such as systemic sclerosis, panarteritis nodosa, etc. as well.22,25
Signs and symptoms of pancreatitis (upper abdominal pain, fever, rapid pulse, nausea, vomiting, weight loss, etc.) may vary, depending on the type of pancreatitis.2 According to Vollertsen and Conn28 the most common laboratory findings of rheumatoid vasculitis are the elevated ESR, increased CRP level, anemia, thrombocytosis, hypoalbuminemia, and a positive rheumatoid factor.28 In Nikolaisen and coworkers’ study29 the cut-off titres for rheuma factor by Waaler-Rose haemagglutination test was 1:160.
Laboratory values mentioned by Vollertsen and Conn28 were elevated in our patient cohort’s with A-SV as well, but probable, because of the limited number of patients and/or the high values of SDs, the differences were not significant. In contrast with these we found very strong and consequent differences between our patient cohort’s regarding the Latex (p<0.0003 and 0.0014), BUN (p<0.011 and 0.018) and creatinin (p<0.0033 and 0.008) between patients with and without A-SV or in comparison with the total population of the patients.
In earlier studies the renal involvement has been considered unsusual in rheumatoid vasculitis, but according to several recent ones this may be more common than previously recognized.28 The higher values of BUN in comparison to normal range (9.14±3.25 versus 2.00-8.90 mmol/l) and the elevated creatinin level (81.2±33.02 versus 62-106 μmol/l) support the renal involvement by A-SV. The significantly lower values of BUN (9.14 mmol/l) and creatinin (81.27 μmol/l) with A-SV in comparison without A-SV (13.41 mmol/l and 131.42 μmol/l) or total population of patients (12.37 mmol/l and 119.96 μmol/l) suggest, that the reduced renal function is caused primarily not by vasculitis. The role of amyloidosis may be more important.
The RA patients were anemic in all groups of patients in comparison to the normal laboratory range (4.05 T/l versus 4.50-5.90 T/l). RBC values decreased in patients without A-SV (3.68 T/l) or in total population (3.78 T/l), and were lower than in patients with A-SV (4.05 T/l) suggesting also, that not the A-SVinfluence primarily the anemia. Renal hypoxia stimulates erythropoiesis in the bone marrow by erythropoietin secreted of the kidneys, and renal hypoxia and may be related to renal amyloidosis as well.
Our study supports the clinical significance of laboratory parameters (elevated erythrocyte sedimentation rate, increased C-reactive protein level, anemia, thrombocytosis, hypoalbuminemia, and a positive rheumatoid factor) mentioned in the literature for the diagnosis of autoimmune systemic vasculitis (A-SV), but significant differences were found only regarding the Latex fixation, BUN and creatinine levels of RA patients. Unfortunately the classic clinical-laboratory parameters (mentioned in the pertinent literature and analyzed in our study (Latex, BUN, creatinine, albumin, alfa-2 globulin, CRP, Waaler-Rose, RBC, and ESR) are not specific for vasculitis and do not predict vasculitis. They are related to the basic activity of RA, to renal complications of RA or to the actual intensity of inflammatory processes of the disease.
In approximately 5-6% of the patients chronic pancreatitis is caused by autoimmune inflammation.7,8 Recognition of autoimmune vasculogenic pancreatitis belongs to the art of medicine. Clinically known autoimmune diseases and clinical suspicion of vasculitis (classic skin lesions: purpura, petechiae, deep cutaneous ulceration, peripheral gangrene, digital or nailfold infarcts), and neurological symptoms (mononeuritis multiplex, peripheral neuropathy) may help and remain the main features in suspected vasculitis, prevailing as leading indicators for its detection.18,30–32 Other causes of similar lesions (diabetes, atherosclerosis, drug reactions, infection, and neoplasm) should be excluded.33 In cases of suspected vasculitis the biopsy remains the gold standard. Because the low incidence of vasculitis in the skin (34.78 %) or the relatively rare involvement of peripheral nerves (52.17 %) we suggested the sural nerve biopsy with surrounding muscle (with 64.29 % prevalence) to confirm and characterize an existing vasculitis (with or without visible involvement of the skin).34
In a cohort study of Chang et al. (2015) the administration of oral corticosteroid decreased the risk of acute pancreatitis in RA patients,1 and according to Mustak et al. (2011) the treatment of autoimmune pancreatitis combined with steroid and metothrexate may also be successful;35 presumably these medications may also be usful in the treatment of aRelLnP generated byA-SV.
The risk of LnP or A-SV is higher in elderly female RA patients, and their chance of survival is lower than in males, or compared with RA patients who did not have LnP or A-SV. A-SV, as a basic complication of RA, should be regarded an important vasculogenic factor in the pathogenesis of aRelLnP, which may be regarded as a special manifestation of autoimmune pancreatitis or a vasculogenic entity in RA.
Our study supports the clinical significance of laboratory parameters (elevated erythrocyte sedimentation rate, increased C-reactive protein level, anemia, thrombocytosis, hypoalbuminemia, and a positive rheumatoid factor) mentioned in the literature for diagnosis of A-SV, but significant differences were found only regarding the Latex fixation, BUN and creatinin levels of RA patients. Unfortunately the classic clinical-laboratory parameters (mentioned in the pertinent literature and analyzed in our study (Latex, BUN, creatinine, albumin, alfa-2 globulin, CRP, Waaler-Rose, RBC, and ESR) are not specific for vasculitis and do not predict vasculitis. They are related to the basic activity of RA, to renal complications of RA or to the actual intensity of inflammatory processes of the disease.
Elevated diastase values were characteristics for LnP, and GGT levels showed significant differences between patients with and without LnP or in comparison to total population. The study is recommended to general physicians, who may meet patients with autoimmune disorders or are interested in autoimmune diseases.
None.
The author declares no conflicts of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.