eISSN: 2373-6372


Crohn’s disease (CD) and tuberculosis (TB) are two granulomatous diseases that can affect the digestive tract. They usually share the same clinical, radiological, endoscopic and pathological characteristics.1 In Morocco, a high endemic area for TB, we observe an increasing prevalence of CD. Therefore, it will be inappropriate and even dangerous to treat a patient who has actually a CD with a potentially toxic anti-Tubercular (anti-TB) therapy or to give immunosuppressive agents to a patient who has TB. We present a case report of a young man who presented with ileo-caecal CD after being treated 1 year earlier for anal tuberculosis.
A 29 years old young man with no previous medical history, presented 2 years ago with an anal abscess and a weight loss of 8 kilograms within 3 months with no bowel movements disorders or blood stool. An acid-fast bacilli stain test of pus sample showed Mycobacterium Tuberculosis organisms. A chest X-ray showed an emphysematous lung, sputum smears and culture were negative. The rectosigmoidoscopy did not show any endoscopic rectal abnormality. The patient had no personal or familiar TB history, he was known to be a smoker. He then received a 6 months’ anti-tuberculosis treatment: 2 months of combined Rifampin (R), Isoniazid (H), Ethambuthol (E), and Pyrazinamide (Z) followed by 4 months of Rifampin (R) and Isoniazid (H). The patient experienced an asymptomatic elevation of liver enzymes (less than 5 times the upper limit), the treatment was then reduced to the minimum effective dose. The monitoring showed afterwards, a normalization of liver enzymes values. During treatment, the patient undergone a fistulectomy, and the pathological specimen did not show any specific abnormality. The patient gained weight, and at the end of treatment the fistulectomy wound healed perfectly. One year later, the patient presented with abdominal pain at the right iliac region and Köenig syndrome, diarrhea more than five times a day and a weight loss of 14 kilograms in 3 months. The clinical examination found a BMI of 19kg/m2 and a sensibility in the right iliac region. An abdominal ultrasound showed a 10 millimeters wall thickening of the terminal ileum, and the blood tests showed a C-reactive protein (CRP) level of 50mg/l, a hemoglobin count of 10g/dl with a low mean corpuscular volum (MCV) and a negative HIV screening. Colonoscopy showed an ulcerated stenosis of the ileocaecal valve, the biopsy specimens showed a chronic mucosal inflammation with no other specific findings. The patient underwent an ileocaecal resection and a termino-terminal ileo-colic anastomosis. The surgical resection specimen showed a chronic architectural distorsion, increased lamina propria plasma cells and lymphocytes, 2 granulomas without caseum, and a fistula extending through all the parietal layers. The anti-saccharomyces cerevisiae antibodies (ASCA) Ig G and Ig A were positive: 300 U/ml and 100 U/ml respectively. The Quantiferon TB gold was, on the other hand, negative (0,01 UI/ml). At 6-months’ post surgery, the patient gained weight (51 kg versus 45kg before surgery), he did not report any abdominal pain or abnormal bowel movement. The blood test showed a CRP level of 0,2mg/l, an albumin level of 42 g/l and a hemoglobin count of 12,6g/dl. The colonoscopy showed 3 ulcerations in the distal ileum (Rutgeerts i1). The ileal biopsies and the random biopsies of the colon did not show any specific finding. Currently, the patient is not taking any treatment and will be seen at 1-year post surgery colonoscopy.(Figure 1-3)
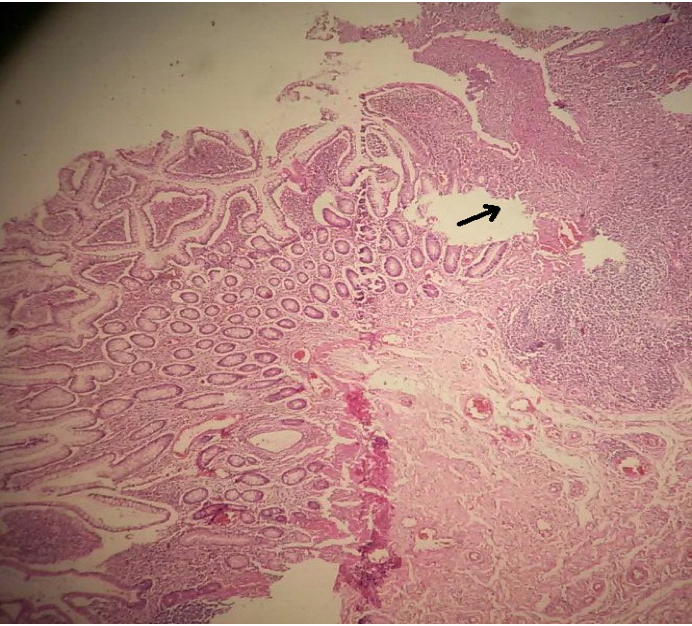
In the TB endemic areas, such Morocco,2 the differential diagnosis between intestinal TB and CD can be often difficult. Even if they do not share the same etiopathogenesis, they usually share the same clinical, endoscopic, radiological and pathogenic characteristics. Therefore, before starting a treatment it is of high importance to correctly distinguish between these two diseases without any delay. In the two situations, weights loss, fever and abdominal pain are usually presents. Anal abscess and fistulas are more often reported in CD.3,4 The symptoms duration seems to be much longer in CD than tuberculosis: 58,1±9,8 months and 7,2±3,4 months respectively.1 The ileo-caecal area is the most common involved region in the two conditions. The endoscopic features are often common such as: pseudopolyps, stenosis and ulcerations.3,5–7 Imaging features such as thickening of the intestinal wall and lymphadenopathy are common to intestinal CD and TB.8,9 A Chest X ray may show a pulmonary TB which is associated with intestinal TB in 32% of cases.10 The pathology specimens can show granulomas for the two diseases but presence of confluent granulomas with caseum is more discriminative for intestinal TB.11 The blood tests can show an anemia, an increased Platelets count, CRP level and sedimentation rate and a decreased albumin level in the two situations. The acid-fast bacilli stain test can identify Mycobacterium tuberculosis organisms in 30% of cases.12 The culture for Mycobacterium Tuberculosis accuracy ranges from 25% to 35% but the results are not available before 3 to 8 weeks. Polymerase chain reaction (PCR) analysis of endoscopic biopsy specimens or pus provides us with a rapid diagnosis within 3 days.13 For our case, we could not unfortunately use this technique for its high cost. The positive acid-fast bacilli stain and the initial response to anti-TB treatment were the criteria that confirmed the diagnosis of anal TB. After receiving the resection pathology results, we asked for ASCA and Quantiferon TB gold testing. ASCA are serological markers particular to CD: a specificity of 83% and a sensibility of 33%, but those markers are not enough to confirm CD diagnostic. Nearly half of TB patients have an ASCA positive.14,15 The interferon Gamma Released Assays (IGRA) consists on detecting the interferon gamma produced by T Cells of patients already exposed to Mycobacterium Tuberculosis, when they are re-challenged with mycobacterial antigens. Initially this test’s utility was to detect latent TB. In a systematic review, we reported that Quantiferon TB gold have a sensibility of 81% and a specificity of 85% for the diagnostic of tuberculosis,16 other studies showed a sensibility, specificity, positive predictive value (PPV) and negative predictive value (NPV) of 84,2%, 74,5%, 50% and 94,2% respectively.4,5,17 Then it is safe to rule out TB if this test is negative. In addition, after an anti-TB treatment the level of an initially positive Quantiferon Tb-gold could decrease but would remain positive at the end of treatment.18 In our case, that test was negative after treatment. A Korean study showed that when ASCA is positive and Quantiferon-TB gold is negative, which is the case for our patient, it had an overall specificity, sensibility, PPV and NPV for CD diagnosis of 97,3%, 41,6%, 93,8% and 63,5% respectively and 96,6%, 23,1%,60% and 84,4% respectively in patients who received anti-TB medical trial.19 With all these facts, what if the patient had actually an anal CD, then, how can we explain the initial improvement under anti-TB treatment. Multiples theories were raised: 1) the 2 conditions involve Paneth cells 2) it will appear that CD is associated with Mycobacterium paratuberculosis 3) the natural pattern of CD is characterized by spontaneous remission phases.20
The differential diagnostic between CD and TB is a daily challenge for gastroenterologists in high epidemic areas. These two conditions share all the clinical, endoscopic, radiological and pathological characteristics but diverge when it comes to treatment. Some specific tests such as ASCA and IGRA seem to help discriminate between these two diseases. But sometimes an anti-TB medical trial is needed. In our case we assist to an association between these two diseases in the absence of an immunodeficiency background.
None.
The author declares no conflicts of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.