eISSN: 2373-6372


A congenital oesophageal malfeasance may be designated as the oesophageal atresia. The persistent condition may be associated to the malady addressed as trachea-esophageal fistula. The disorder was detected three millenniums ago. An effortless reconstruction of the deformity may be achieved by virtue of the developments within the neonatal intensive care units (NICU), the employment of parenteral nutrition, superior surgical procedures and antibiotics besides enhanced and appropriate prenatal interpretation of the condition. The aforementioned measures may assist in curtailing the infant morbidity and mortality.1
The oesophagus and trachea evolve from the foregut bud. A tracheal ventral diverticulum of the foregut bud emerges amidst the fourth week of embryogenesis. The septate, heteromorphic laryngo-tracheo-esophageal groove connects the trachea and the oesophagus. The distal oesophagus, the heart and the lung usually evolve by the seventh week. The proximal third of the oesophagus with striated muscle originates from the pharyngeal pouch. The blood circulation is supplied from the thyrocervical trunk with and coexistent submucous blood vessels. The smooth muscle of the middle and distal oesophagus emerges from the dorsal mesenchyme.1,2 The circulatory component derived from the segmental roots of the aorta may not be amenable to appropriate division and ischemia ensues.3 The atretic oesophagus configures a blind protrusion and the distal tracheo-oesophageal fistula progresses as a middle branch of the tracheal trifurcation. The distal foregut analage transforms into a pulmonary phenotype, trifurcates and the middle branch expands caudally to dilate into the stomach. The distal analage produces the ectopic oesophageal lungs with a defective respiratory movement and a histological evidence of the airway cartilage within the lungs.2,4 Frequent aberrations manifested may be i) a proximal oesophageal atresia appended to the distal tracheo-oesophageal fistula (87%). The amniotic fluid pressure may enlarge the hypertrophic proximal oesophagus. The trachea encroaches upon the inflated pouch to create a defective cartilaginous ring, the tracheomalacia. ii) a pure oesophageal atresia (8%) which may be accompanied by a deficient distal oesophagus. iii) an isolated tracheo- oesophageal fistula is may be discerned as the third common variant. Adjuvant derivatives detected are inconsequential and may be identified intra-operatively.1
A male predominance and a frequency of one in every 2500-5000 live births may be encountered.2 A pregnancy numero uno, elderly patients, diabetic matriarchs, twin pregnancy, chromosomal aberrations and hereditary conditions tend to exacerbate the possibility of the disorder.1,2 Protracted oestrogen, progesterone or thalidomide administration during the pregnancy may be teratogenic. Chromosomal aberrations may induce a Trisomy 13, 18 (Edward’s syndrome), 21 (Down’s Syndrome) or the VACTERL association, which may predispose to the disorder.4 Besides, a deletion of band 11.2 of the long arm of chromosome 22 along with the long arm of chromosome 17q22-q23.2 may be exemplified with conditions such as oesophageal atresia, tracheo-oesophageal fistula and conductive hearing loss.5 Oesophageal atresia appears within a short period after birth. Clinical aspects of excessive mucus/salivation, reflux after the primary feed, cough, asphyxia, cyanosis, respiratory distress or failure to insert an oro/ nasogastric tube may be enunciated. A scaphoid or a tumescent abdomen may be demonstrated in a distal tracheo-oesophageal fistula.
The prenatal detection of oesophageal atresia on an ultrasound may be defined as a miniscule or an imperfect foetal stomach bubble. The positive predictive value of the evaluation may be configured at 56% and the sensitivity at 42%.1 The feature may be in concurrence with the presence of maternal polyhydroamnios.5,6 Similarly, the emergence of pure oesophageal atresia may be concomitant with maternal polyhydramnios. A delineation of the prenatal blind oesophageal stump during foetal swallowing may be designated as the pouch sign. Prenatal investigations may be accompanied by ambiguous values of maternal alpha fetoproteins. A coiling oro/nasogastric tube within the proximal oesophagus, as elucidated on a plain x-ray chest, may be corroborative of an oesophageal atresia., Infrequently indicated contrast studies may be catastrophic on account of the risk of aspiration of contrast material. X-ray films of the abdomen may be obtained in order to exclude coincident gastrointestinal anomalies. Additional diagnostic procedures may be an Oesophagoscopy, Fluoroscopy and Bronchoscopy. Appearance of air in the gastrointestinal tract may imply the presence of a distal tracheo-oesophageal fistula, whereas the lack of air may allude to an oesophageal atresia. Infants with oesophageal atresia may require a baseline assessment for concomitant congenital malformations, an electrocardiogram for cardiac duct dependent anomalies, a preoperative renal ultrasound in the absence of voiding and a comprehensive assessment of the chromosomal anomalies.6 Isolated tracheo-oesophageal fistula may be considered as a congenital malfeasance dependent upon intricate modalities of identification and treatment. The oblique, fistulous trachea arising at the level of the tracheal neck root C7-T1 may extend up to the carina or main bronchus. In contrast to the H type, the frequent type N of trachea-oesophageal fistula favours the bilateral interchange of air and oesophageal constituents on account of the altered air pressure. Predominant systemic symptoms may be cyanosis, coughing, choking while feeding, repetitive chest infections, incessant gastrointestinal dilatation and sporadic hyper salivation.3,7 An oesophagogram or a video oesophagogram may be confirmative of the condition and appears beneficial in assessing the level of the fistula. Instances of equivocal constitutional symptoms or diagnostic parameters may necessitate an adjunctive bronchoscopic evaluation. Deferment of the clinical diagnosis and debatable systemic manifestations may impede an appropriate and timely surgical intervention (Figure 1-5).
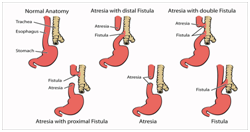
Figure 1 Variants of TEF.12
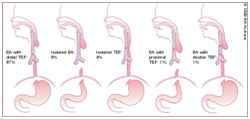
Figure 2 TEF sub-categories.13
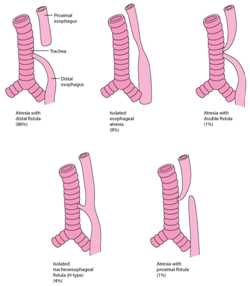
Figure 3 Percentage proportions of TEF.14
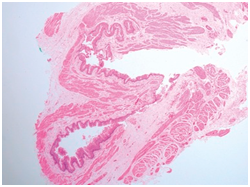
Figure 4 TEF with muscular hypertrophy and atrophic mucosa.15
Figure 5 TEF: Squamous epithelium in respiratory tract.16
The subdivision of probable complications, as described with the Waterston projections in 1962 and re-affirmed in 1994, may be considered timeless with a universal application.8
Group I |
Birth weight >1500 gm without major cardiac anomaly. |
97% survival |
Group II |
Birth weight <1500 gm or major cardiac anomaly |
59% survival |
Group III |
Birth Weight <1500gms and major cardiac anomaly. |
22% survival |
Oesophageal atresia may coexist with severe systemic aberrations (50-70 %) in order to characterize distinctive clinical and pathological manifestations. Cardiovascular anomalies (29%, genitourinary inconsistencies (14%), gastrointestinal incongruities(13%), skeletal deviations (10%) and chromosomal deficiencies (4%) may modify the disease prognosis or survival outcomes. Abnormalities such as neural tube defects, hydrocephaly, holoprosencephaly and macro-opthalmia may be synergistic with the disorder. However, anomalies such as choanal atresia, cleft lip, abdominal wall deficiency and diaphragmatic hernia may appear sporadically with the disease. The VACTERL association (1973) annotates the emergence of oesophageal atresia with adjunctive congenital/neonatal disorders in an estimated 14% to36% instances. The following diseases may be elucidated:
i)Vertebral Anomalies, ii) Anal Malformations, iii) Cardiac Malformations, iv) Tracheo-oesophageal Fistula, V) Renal deformities, vi) Limb radial defects alternatively, additional disorders which may be affiliated with oesophageal atresia may be defined by the CHARGE syndrome: Coloboma, Heart disorders, Retarded development or growth, Central nervous system anomalies, Genital hypoplasia or hypogonadism, Ear abnormalities or deafness. Apart from the aforementioned conditions, symptomatic triads such as Down’s syndrome, Potter’s syndrome, Fryn’s syndrome and Fanconi’s syndrome may be exemplified.7 Infants born with oesophageal atresia may depict cardiac malformations (30%) which may predominantly be duct dependent lesions (Tetralogy of Fallot, pulmonary atresia, tricuspid atresia, transposition of great arteries, patent ductus arteriosus, ventricular septal defect or anomalies of the aortic arch).1,2 Instances of tracheo-oesophageal fistula accompanied by the non duct dependent cardiac defects may mandate a preferred reconstruction of the oesophagus. However , oesophageal atresia associated with duct dependent cardiac lesions may be temporarily managed with the administration of Prostaglandin E, preceding the surgical closure, in order to address the cardiac anomalies foremost. Anomalies such as hypospadias, undescended testis, renal agenesis, hydronephrosis, vesicouretral reflux and ambiguous genitalia may outline the spectrum of coexistent genito-urinary malformations. Gastrointestinal malfeasance usually constitutes of an imperforate anus, duodenal atresia, malrotation of the gut, annular pancreas and pyloric stenosis. Anomalisms of the respiratory system may elucidate an oesophageal atresia, ectopic superior left bronchus, a trifurcated trachea or tracheal bronchi, congenital bronchial stenosis or the emergence of tracheal, bronchial and pulmonary agenesis.6 Respiratory abnormalities with predominant tracheomalacia and pulmonary atelectasis may necessitate an early bronchoscopic evaluation. Chromosomal defects such as a Trisomy 18 and 21 may infrequently concur. The surgical closure of oesophageal atresia when appearing concomitantly with Trisomy 18 may require approval from an ethics committee.
As the malfeasance of oesophageal atresia is not categorized as a surgical emergency, the measures to be incorporated in the pre-operative management of the condition may be i) protection of the airway, circumvention of the aspiration and treatment of the accompanying pneumonitis, if required. An echocardiogram may be a pre-requisite to localize the position of the aortic arch. Preterm babies elucidating symptoms of respiratory distress due to hyaline membrane diseases in conjunction with oesophageal atresia may mandate the employment of mechanical ventilation. In the event of incompetent ventilation from the oesophageal defect, a gastric perforation, abdominal compartment syndrome or hypoxia may ensue. A substitute, emergent ligation of the trachea-oesophageal fistula may be considered the in such exterminating circumstances.
Intra-operative measures
A minimally invasive thoracoscopic reconstruction, as exemplified in 2002, may be employed, in contrast to a conventional open reconstruction of the defect.9 Short term surgical outcomes such as the time required in surgical closure, post operative leaks and strictures rates may be equivalent with the dual techniques. The kind of deformity and the concordant congenital malfeasance usually dictates the intra-operative standard of care. Continuity of the bowel may be a necessary aspect of the therapeutic protocol.
Surgical repair
Post –operative measures
Mechanical ventilation and prophylactic paralysis of the neck muscles besides gavage feeding as directed by the trans-anastomotic tube may be considered as beneficial methodologies in the post –operative period to aid the healing of the surgical defect. A barium swallow may be employed within a week to ten days in order to discern a leak, stricture, dysmotility or a gastro-oesophageal reflux.2,4
Initial as well as eventual morbidity may limit the outcomes of the surgical restoration of oesophageal atresia and tracheo- oesophageal fistula. Frequent and complex ramifications of the applicable anastomosis may be delineated as a stricture (50%), leakage (10-15%) or a re-occurrence of the tracheo-oesophageal fistula (3-15%).1 Gastro-oesophageal reflux (GER), tracheomalacia and dysmotility may configure as delayed repercussions. The development of strictures or stenosis may be accompanied with choking, continual respiratory infections or may incite a foreign body impaction. Since a second, consecutive or subsequent thoracotomy may be disastrous, additional techniques to approximate the tracheo-oesophageal fistula may be employed.5,6 The particular procedures comprise of endoscopic diathermy, obliteration, coagulation, endoscopic chemo-cauterization, laser methodologies or a fibrin glue deposition. Reconstruction of an oesophageal atresia may induce a gastro-oesophageal reflux, oesophageal shortening, denervation of the vagus nerve, progression of the stricture with inflammation and oedema, recurrent pneumonia, failure to thrive, conversion to a Barrett’s epithelium and severe oesophagitis. The aforementioned complications may be managed by an adequate fundoplication.1 A structural or a functional, generalized or localized, deficiency of the tracheal rings may ensue and may be ascribed to as Tracheomalacia. It may account for a partial respiratory obstruction. Classically, it manifests as a harsh, barking cough and malnutrition may ensue when cyanosis occurs while feeding. The particular condition may elucidate a narrow slit like appearance which may be identified with a plain, lateral thoracic x-ray. A bronchoscopy may similarly be employed during spontaneous breathing (as an antero-posterior narrowing occurs during expiration) along with a cine tracheo-bronchoscopy (to detect the magnitude of tracheal disintegration ) and a cine computerized tomography to discern the tracheal collapse. Aortopexy may be recommended for tracheomalacia. If inadequate a tracheal augmentation may be achieved with autologous cartilage grafts.2
The preceding half a century has witnessed an elevation in the survival rate of infants with oesophageal atresia, in the absence of severe coexistent deformities to the current estimated figures of 80-95%. However, inferior outcomes may be witnessed in patients with chromosomal aberrations, infants on ventilators and those with an extensive tracheo-oesophageal defect. Impairment of respiratory and gastrointestinal functions may be elucidated and commonly constitute of dysphagia, dyspnoea and persistent respiratory infections with nocturnal cough (30%) and a gastro-oesophageal reflux (20%). The preceding developments may deteriorate the quality of life. Nevertheless, the intensity of the constitutional symptoms may diminish in adulthood. Concomitant aspects such as gastric metaplasia (premalignant), oesophagitis, intense tracheal, oesophageal and gastric inflammation may require an extended follow up.10 In contrast to a primary and competent surgical closure, procedures such as an oesophageal replacement may produce unsatisfactory results as the babies tend to manifest anastomotic strictures, ulceration, haemorrhage, redundancy and engorgement of the stump with bezoar.10,11
None.
The author declares no conflicts of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.