eISSN: 2373-6372


The prevalence of obesity is rising worldwide and is a major risk factor for a various cardiometabolic diseases and some malignancies. Obesity is usually defined by BMI, however other methods are used to evaluate more precisely the visceral obesity due to its inevitable link to increased morbidity and mortality. In this review we approached sarcopenia in the context of obesity and metabolic syndrome to overview the recent research in sarcopenic obesity interventions and benefits of different approaches. We focused on Vitamin D, visceral obesity and NAFLD aiming to summarize the research over last years. Sarcopenia is a risk factor for fatty liver disease. We proposed an integrated, complex and multidisciplinary approach to intervene the nutritional and behavioural deficiencies in development of metabolic syndrome and related visceral obesity in sarcopenic obese people with NAFLD.
Keywords:sarcopenia, obesity, vitamin d, NAFLD, metabolic syndrome
The prevalence of obesity has been steadily increasing over the past 30years. The worldwide prevalence of obesity nearly doubled between 1980 and 2008. According to country estimates for 2008, over 50% of both men and women in the WHO European Region were overweight, and roughly 23% of women and 20% of men were obese. In European Union countries, overweight affects 30-70% and obesity affects 10-30% of adults. What is more alarming is that childhood obesity rates also rising, which is strongly associated with risk factors for cardiovascular disease, type 2 diabetes, orthopedic problems, mental disorders, etc.1 Above 18% of the population of the OECD countries are obese. Nearly one in three is obese in the US and New Zeland, one in four in Mexico and Hungary, Canada and UK, Luxembourg and Czech Republic.2
Obesity is a major risk factor for complications such as type 2 diabetes, non-alcoholic fatty liver disease, dyslipidemia, hypertension, cardiovascular disease, sleep apnea, and gall bladder disease.3 There is also a strong relationship between obesity and the incidence of some types of cancer.4 A different phenotype is described, called metabolically healthy obesity. It is defined as body mass index (BMI)≥30kg/m2 without the presence of metabolic diseases such as type 2 diabetes (T2DM), dyslipidemia or hypertension or less than two cardiometabolic disorders. It is not yet all clear whether this phenotype decreases morbidity and mortality associated with obesity. A study reported that 42% of their subjects with initially metabolic healthy obesity actually developed the metabolic syndrome within 10years, again suggesting the overall increased health risks of obesity.5 Patients with obesity initially defined as metabolically healthy were found to have a higher prevalence of hepatic steatosis as compared to people with normal bodyweight. The highest prevalence of fatty liver disease was recorded in the obese persons with metabolic syndrome. This suggests that obesity per se in absence of metabolic risk factors is not entirely benign and is in fact associated with subclinical vascular inflammation.6
Vitamin D deficiency is increasingly reported in different conditions and diseases. An overall estimation showed that 40.4% of above 50 thousand europeans sampled, irrespective of age group, ethnicity, and latitude of residence had serum 25(OH)D concentrations below 50nmol/L on average in the year.7 Here we present some thoughts regarding the relationships between obesity, sarcopenia and vitamin D deficiency and its relationship with fatty liver disease.
The most frequent definition for overweight and obesity is the one that use the Quetelet's formula or the body mass index (kg/m2). Overweight person is defined as having BMI greater than or equal to 25; and obesity is a BMI greater than or equal to 30.8,9 While BMI is a simple measure that is very useful for populations, it should be considered a rough guide for predicting risk in individuals. The distribution and amount of body fat are also crucial determinants of some obesity-associated health risks. Accordingly, measures of central obesity such as waist to hip ratio (WHR) and waist circumference (WC) provide more robust indices of overall obesity-related health risk than BMI alone. It is suggested that WC or the WHR, which better reflect visceral fat, may be more useful as they are better predict cardiovascular disease (CVD) and mortality. Central obesity has been defined as a WC>102cm for men and >88cm for women, or a waist:hip ratio ⩾0·9 for men and ⩾0·85 for women.10 Another useful index is waist to hejght ratio (WHtR). A study showed that WHtR cutoff of 0.578 (Youden Index=0.50) predicts well metabolic syndrome both in men and women. Participants with a WHtR greater than the cutoff were almost ten times more likely (OR = 9.8) to have metabolic syndrome than those that were not.11
There are other measurements of adiposity (e.g., bioelectric impedance, air/water displacement plethysmography, or dual-energy X-ray absorptiometry, DEXA). As recommended by the Clinical practice guideline of the American College of Endocrinology, these methods might be considered at the clinician’s discretion if BMI and physical examination results are equivocal or require further evaluation. The clinical utility of these measures is limited by availability, cost, and lack of evidences and outcomes data for validated cutoff points.12 Additional methods for evaluation of the body composition and visceral obesity are computer tomography (CT), magnetic resonance MRI, ultrasound. For research purposes a bundle of indices, derived from this methods are used - FMI (fat mass index), FFMI (fat-free mass index), ALM (appendicular lean mass), ALM/BMI, ALM/H2 (ALM-height ratio). For comprehensiveness of our review we will here summarize the used definitions of metabolic syndrome (Table 1).
|
WHO13 |
EGSIR Definition |
NCEP ATP |
IDF |
|
Glucose intolerence, impaired glucose tolerance or diabetes mellitus, and/or insulin resistance, with two or more of the following: |
Elevated plasma insulin (>75th percentile) plus two other factors from the following: |
Three or more of the following criteria: |
Central obesity plus 2 of the following: |
|
Arterial pressure ≥140/90 mm of Hg |
Abdominal obesity: waist circumference (WC) ≥94 cm in men and ≥80 cm in women |
Abdominal obesity: WC ≥102 cm in men and ≥88 cm in women |
The WC cut-off are >80 cm for females and >94 cm for males (>90 for South Asian, Chinese and Japanese) |
|
Plasma triglyceride ≥ 150 mg/dl and/or HDL-C <35 mg/dl in men and <39mg/dl in women |
Hypertension: ≥140/90 mm of Hg or on antihypertensive treatment |
Hypertriglyceridemia: ≥150 mg/dl (1.695 mmol/l) |
raised triglycerides: ≥150 mg/dl (1.7 mmol/l) or history of specific treatment for this lipid abnormality |
|
Central obesity, WHR >0.9 in men and >0.85 in women and/or body mass index (BMI) >30kg/m2 |
Elevated triglycerides (≥150 mg/dl) and/or reduced HDL-C (<39mg/dl for both men and women) |
Low HDL-C: <40 mg/dl in men and <50 mg/dl in women |
reduced HDL cholesterol: < 40 mg/dl (1.03 mmol/l) in males and < 50 mg/dl (1.29 mmol/l) in females or specific treatment |
|
Microalbuminuria, urinary albumin excretion rate ≥20 μgm/minute or albumin/creatine ratio ≥30 μgm/mg. |
Elevated plasma glucose: impaired fasting glucose (IFG) or IGT, but no diabetes |
High blood pressure (BP): >130/85 mmHg |
raised blood pressure: systolic BP ≥130 mm Hg or diastolic BP ≥85 mm Hg or on treatment |
|
High fasting glucose: >110 mg/dl |
raised FPG: ≥ 100 mg/dl or previously diagnosed type 2 DM |
Table 1 Definitions of metabolic syndrome13–15
The American Association of Clinical Endocrinologists (AACE) preferred using the term insulin resistance syndrome over MS.16 The major criteria they considered were IGT, elevated triglycerides, reduced HDL-C, elevated BP, and obesity. No particular number of criteria for diagnosis is specified, but it is left on clinical judgment. Factors like family history of atherosclerotic cardiovascular disease or type 2 DM, polycystic ovary syndrome, and hyperuricemia should be considered as well as degree of glucose intolerance, dyslipidemia, renal sodium retention, hypertension, prothrombotic factors, systemic inflammation and endothelial dysfunction. Abdominal obesity is the most prevalent form of the metabolic syndrome. It has been found to be a major correlate of a cluster of diabetogenic, atherogenic, prothrombotic and proinflammatory metabolic abnormalities referred to as the metabolic syndrome.17
None of these definitions actually cover the liver involvement. For the last decade however, a huge amount of research was focused on NAFLD (Non-alcoholic fattly liver disease) as a feature of insulin resistance and MS. Available data from clinical, experimental and epidemiological studies indicate that NAFLD may be the hepatic manifestation of metabolic syndrome.18 Visceral fat, particularly in the abdominal region, has a stronger association with type 2 diabetes and cardiovascular disease than BMI. Recent study found very strong correlation between visceral fat deposits and fatty liver disease. Subjects were initially diagnosed with NAFLD by combination of ultrasound and MRI. All obesity measures, except for subcutaneous fat area (SFA), were higher in subjects with NAFLD compared to controls. More, subjects with increased waist to hip ratio (WHR) were three times more likely to have a fatty liver.19 Visceral adiposity strongly correlated with ultrasound and MRI diagnostic features of NAFLD. This study also showed poor relationship between subcutaneous fat and fatty liver disease.
NAFLD, in the presence of normoglycemia and normal or moderately increased body weight, is characterized by clinical and laboratory data similar to those found in diabetes and obesity. Insulin sensitivity was studied in 30 subjects with biopsy-proven nonalcoholic fatty liver disease (NAFLD), normal glucose tolerance, and a BMI <30 kg/m.2 Most NAFLD patients had central fat accumulation, increased triglycerides and uric acid, and low HDL cholesterol, irrespective of BMI. There was a reduced glucose disposal to an extent similar to that of the type 2 diabetic patients. Compared with type 2 diabetic patients, NAFLD patients had lower basal hepatic glucose production (HGP) but with similarly reduced insulin-mediated suppression of HGP. NAFLD may be considered an additional feature of the metabolic syndrome, with specific hepatic insulin resistance.18 The role of central adiposity seems crucial in the fatty liver disease patophysiology. Visceral fat is an important source of triglycerides leading to steatosis. This probably explains the presence of generally lean, centrally obese individuals with NAFLD.20
Some data suggests that fat deposition in the liver cells might be secondary to a primary skeletal insulin resistance. The mechanism(s) responsible for the marked differences in the relationship between hepatic steatosis and insulin resistance across different studies is not known. It is suggested that other steatogenic factors such as inflammation, circulating adipokines, ER stress, or as yet unidentified lipid metabolites, affect insulin sensitivity but are not necessarily directly related with intrahepatic triglyceride content.21 Different cytokines and adipokines like leptin, TNF-a, IL-6, etc. has role in development of the NAFLD in metabolically obese patients. In obese NAFLD patients, leptin levels are elevated and are directly correlated with the severity of steatosis. The presence of hepatic steatosis despite the presence of hyper-leptinemia suggests the development of leptin resistance.22
Sarcopenia is defined as a normal decline of the muscle mass and strength with aging. It is however recognised recently as a distinct condition and included in the IDC-10th revision. Currently, there are three consensus papers, published by the European Working Group on Sarcopenia in Older People (EWGSOP), the European Society for Clinical Nutrition and Metabolism Special Interest Groups (ESPEN-SIG), and the International Working Group on Sarcopenia (IWGS). All of them define sarcopenia as a presence of low skeletal muscle mass with either low muscle strength or low muscle performance. When all three conditions are present, severe sarcopenia may be diagnosed (EWGSOP). According IWGS definition sarcopenia is associated with muscle mass loss alone or in a conjunction with increased fat mass.23–27 According to the EWGSOP definition ‘presarcopenia’ is characterised by low muscle mass without impact on muscle strength or physical performance. This stage can only be identified by techniques that measure muscle mass accurately and in reference to standard populations.23 The Society of Sarcopenia, Cachexia and Wasting Disorders defined ‘sarcopenia with limited mobility’ as lean appendicular mass/height2 of two SDs or more below the mean for 20-30year olds, with a walking speed of ≤1m/s.25 Sarcopenia increases from 14% in those aged 65 to 70years to 53% in those above 80 years of age. Even with a conservative estimate of prevalence, sarcopenia affects >50 million people today and will affect >200 million in the next 40years. The impact of sarcopenia on older people is far reaching related to morbidity, disability, high costs of health care and mortality.28 Sarcopenia however affects not only aging populations. It is a syndrome, which develops in the context of variety of chronic diseases, including cancer, chronic kidney disease, etc.
Sarcopenic obesity (SO) is considered to be a combination of decreased muscle mass and function and excess body fat mass. However, considering the previously mentioned problems regarding criteria for obesity and sarcopenia alone, it is no surprise that a widely accepted specific definition of SO to date does not exist. The prevalence of SO in different studies is reported to range from 4% to 84% in men and from 4% to 94% in women, depending on the definition employed. While most of definitions for SO focus on muscle mass alone and few consider functional criteria further confusion to the already heterogeneous field of SO definitions and cutoff values contributes to the poor consistency of results.29 The extent of visceral obesity was shown to correlate with the extent of loss of appendicular muscle over ~2years of follow-up, indicating that there may be a causal component to this association.30 The insulin resistance in the muscle is a key link between obesity and metabolic syndrome. Sarcopenic obesity actually is the condition, which links obesity, IR and sarcopenia. It is believed that sarcopenic obese individuals are at an increased risk of adverse health events compared with those who are obese or sarcopenic alone.31 Normally elderly persons lose anabolic insulin effect, while they are sensitive with the regard of the glucose. On the contrary , obese subjects represent an increase in the intramyocellular lipid deposition related to impaired protein synthesis and glucose metabolism. Sarcopenic subjects with visceral obesity have increased numbers of intermuscular adipocytes which can further produce prodiabetic cytokines.32 Some authors reported the potential roles of rapamycin signalling, AMP-activated protein kinase, myostatin, urocortins and vitamin D in the pathogenetic mechanisms. Sarcopenic obesity could be associated with higher levels of metabolic disorders and increased risk of mortality than obesity or sarcopenia alone. Efforts to promote healthy ageing should focus on both preventing obesity and maintaining or increasing muscle mass.
There is a new research interest in the role of the vitamin D in obesity and related metabolic disturbances. Animal studies showed that deficient state leads to impaired muscular strength.33 However studies in humans are contradictory. Several studies from Korea examined the relationship between body composition and variety of cardiometabolic risk factors including blood pressure (BP), glucose tolerance indices, lipid profiles, inflammatory markers, and vitamin D level. The prevalence of vitamin D deficiency and metabolic syndrome was highly prevalent in the sarcopenic obese group. The sarcopenic obese patients was more closely associated with insulin resistance, metabolic syndrome, and CVD risk factors than normal or obese elderly patients.34 Another study measured visceral obesity and its relationship with Vitamin D in sarcopenic obese patients. Interestingly, the SO women had higher HOMA-IR (Homeostatic Model Assessment of Insulin Resistance) and hsCRP levels (highly sensitive C-reactive protein). In men, vitamin D levels were lower in the SO group than the non-SO group. Both hsCRP and HOMA-IR levels positively correlated with visceral fat area in both men and women, whereas vitamin D levels positively correlated with skeletal muscle mass index in both men and women. HOMA-IR and vitamin D levels were independently associated with sarcopenic obesity in men, while HOMA-IR and hsCRP predicted SO in women.35 In elderly population showed that vitamin D was in negatively associated with the systolic pressure, visceral obesity and body fat percentage in men and with WC and visceral obesity in women. The mean 25(OH)D level was lower in men with visceral obesity but was not affected by the presence or absence of sarcopenia.36 Very recent study in almost six thousand persons above 50years old found that participants in the highest tertile group of serum vitamin D levels, compared with those in the lowest tertile, were less likely to have osteosarcopenic obesity (OR = 0.42 for men and 0.55 for women). Vitamin D deficient patients were two times more likely to have osteosarcopenic obesity. That study reveals additional health benefits of maintaining adequate levels of vitamin D, especially in obese sarcopenic patients.37
Non-alcoholic fatty liver disease is accepted as one of the features of metabolic syndrome closely correlates with visceral fat tissue accumulation. The role of the skeletal muscle in NAFLD remains unclear. A large study with 11 116 participants (1 848 of them with NAFLD) reported inverse association between the fatty liver and skeletal muscles. Higher skeletal muscle mass may have a beneficial effect in preventing NAFLD.38 Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis as shown by Koo et al. In a biopsy-proven NAFLD cohort the prevalence of sarcopenia in subjects without NAFLD was 8.7%, in those with non-alcoholic fatty liver disease (NAFLD) was 17.9% and in patients with steatohepatitis (NASH) was 35.0%. The appendicular skeletal muscle mass (ASM)/body weight, ASM% was inversely correlated with the severity of fibrosis. More, the prevalence of significant fibrosis was higher in subjects with sarcopenia than in those without it (45.7% vs. 24.7%). Among NAFLD subjects, sarcopenic subjects were at least two times more likely to have NASH than those without sarcopenia after being adjusted for age, gender, BMI, hypertension, diabetes, smoking status and insulin resistance. Sarcopenic individuals were twice times more likely to have significant fibrosis independent of BMI and insulin resistance. This is the first study to show that low muscle mass is associated with significant fibrosis regardless of obesity, inflammation, and insulin resistance.39 Low skeletal musculature was associated with NAFLD independently of other metabolic and lifestyle parameters in both genders. The magnitude of this association was higher in middle aged men and premenopausal women.40
NAFLD and sarcopenia may share pathophysiological mechanisms, such as insulin resistance, inflammation, vitamin D deficiency, and decreased physical activity. The Korean Sarcopenic Obesity Study is a prospective observational cohort study. NAFLD was diagnosed by the liver attenuation index (LAI), measured on CT. Sarcopenia was defined by a skeletal muscle mass index (SMI) measured by DEXA. After adjusting for age and sex, both SMI and LAI were negatively correlated with HOMA-IR and hsCRP as well as arterial stiffness. Both SMI and LAI had positive relationships with HDL-cholesterol, and negative relationship with triglyceride, alanine aminotransferase (ALT), and total body fat. The lowest was SMI, the higher was odd for NAFLD even after adjusting for confounding factors. Individuals with lower muscle mass exhibited increased risk of NAFLD.41
Sarcopenia is а common feature of end stage diseases and liver disease is not ан exclusion. Interestingly and in support of the above mentioned relationship between NAFLD, visceral obesity and sarcopenia, there are evidences that even in cirrhotic patients, sarcopenia is associated in larger degree with NAFLD. Among 207 cirrhotic who underwent liver transplant 20% were with NASH. Sarcopenia was found in 60% of patients prior to the transplant and 40% were with sarcopenic obesity. The last persisted post-transplant. Obesity and age were found to be independently associated with pretransplant sarcopenia but NASH as cause of cirrhosis was independent predictor of sarcopenic obesity (OR 6.03).42
Many studies demonstrated an inverse correlation between serum 25(OH)D and diabetes, metabolic syndrome, insulin resistance and beta cell function. Meta-analyses and cross-sectional studies show inverse correlation between serum vitamin D levels and metabolic syndrome. Prospective studies also support those associations, sugessting dose-depending manner.43,44 Featuring metabolic syndrome, fatty liver disease is also a player in the vitamin D game. Chinese authors showed an inverse association between vitamin D levels and liver fat content in middle aged and elderly men.45 The age- and sex-adjusted prevalence of NAFLD decreased steadily with increase of vitamin D levels. The serum level of vitamin D, even when within the normal range, was found to be inversely correlated with NAFLD in a dose-dependent manner, independently of known metabolic risk factors. It might be speculated that vitamin D exerts protective effects against NAFLD.46.Meta-analysis found that NAFLD patients have decreased serum 25(OH)D concentrations, suggesting that vitamin D may play a role in the development of NAFLD. Demonstration of a causal role of hypovitaminosis D in NAFLD development in future studies could have important therapeutic implications.47 More, vitamin D deficiency increases risk of inflammation and fibrosis in patients with NAFLD.48 Vitamin D deficiency was present in 55% of subjects and was independently associated with definitive NASH, increased lobular inflammation, more ballooning and the presence of fibrosis. It is likely that the activation of the mitogen-activated protein kinase and nuclear factor-κB pathways are engaged in vitamin D deficient NAFLD patients. These findings support dietary and/or lifestyle modifications to increase vitamin D levels in NAFLD patients.
Progression of NAFLD to more aggressive steatohepatitis which have worse prognosis is also vitamin D dependent. A study in morbidly obese patients showed that increased fibrosis is primarily associated with higher HOMA-IR, procollagen type I propeptide (P1NP), lower osteocalcin, albumin-corrected calcium, parathyroid hormone, vitamin D, male sex, and higher age. Pateints with metabolic syndrome and diabetes were 9 to 13times more likely to have advanced fibrosis in the liver.49 Some studies however failed to prove the predictive role of vitamin D levels for advanced fatty liver disease. Study performed in almost 400 morbidly obese patients showed prevalence of NASH of 20.6%. but with a weak correlation between serum levels of vitamin D and fibrosis. More, only steatohepatitis was found to predict the severity of fibrosis.50
Vitamin D deficiency role can be suggested as weight loss per increased serum vitamin D level improved metabolic parameters in NAFLD even without vitamin D supplementation. Liver enzymes and metabolic parameters also improved, even as vitamin D intake decreased in a study with newly diagnosed fatty liver patients. Serum vitamin D concentration increased with body weight and intrahepatic fat reduction, independent of decreases in vitamin D intake.51 A possible explanation for the effects of vitamin D in fatty liver disease is the hepatocyte vitamin D receptor, which regulates the lipid metabolism. Mouse models were used to demonstrate that expression of vitamin D receptor is markedly induced in NAFLD. Vitamin D receptor deletion in high fat diet-fed apoE(-/-) mice protected against fatty liver, dyslipidemia and insulin resistance, and caused a decrease in taurine-conjugated bile acids, but did not influence fibrosis by thioacetamide.52 Likely, the vitamin D receptor can contribute to the fatty liver disease promoted by a high fat diet.
Sarcopenia is an important health issue of the twenty-first century both because its increasing prevalence among elderly and middle aged population and increasing healthcare costs for treating its consequences. They include an increased falls risk, a decreased physical ability and quality of life and an independent increase of all-cause mortality. At the moment there is no licenced treatment for sarcopenia, including its phenotypes like sarcopenic obesity. The treatment approach has to be multidisciplinary and complex. It has to include the input of different medical specialties like geriatricians, nutritionists, endocrinologists, gastroenterologists, occupational therapists and kinesitherapists (Figure 1).
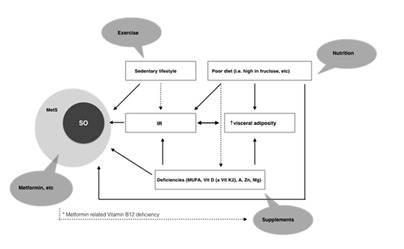
Figure 1 Relationship between risk factors and development of sarcopenic obesity and metabolic syndrome. Various interventions are proposed for each risk factor.
In the complex of lifestyle changes a place of the exercise should be highlighted.53 A moderate energy restriction, targeted at a moderate weight loss of 0.5-1kg/wk or while assuring a protein intake of at least 1g/kg body weight seems the best nutritional advice for the long term.54 It is worth to mention that so called yo-yo effect will even advance sarcopenic obesity causing regain of fat mass. Combination of essential amino acids also show benefits (i.e. a leucine-rich balanced essential AA mix.55 Other nutritional supplements that have been tested and previously reviewed like creatine, β-hydroxy- β-methylbutyrate (β-HMB, a leucine metabolite), the AA arginine and β-alanine, omega 3 fatty acids, and a number of antioxidant nutrients including carotenoids, selenium, vitamins E and C, and isoflavones.29
Quite a few studies shows benefit from supplementing vitamin D in sarcopenic patients. A study in postmenopausal women showed that 9months of supplementation with vitamin D3 1000 IU/day increased serum levels by 45.4% with significant increases in muscle strength and control of progressive loss of lean mass.56 Vitamin D supplementation has been shown to decrease insulin resistance. Recent study demonstrated the effect of vitamin D supplementation on blood sugar and different indices of insulin resistance in patients with non-alcoholic fatty liver disease (NAFLD). Patients received vitamin D3 (50,000 IU/week). Intake of vitamin D supplements led to decrease in fasting blood glucose and HOMA-IR in patients with NAFLD.57 The nutraceutical armamentarium for NAFLD includes mainly antioxidants vitamin E, polyphenols which reduce liver fat accumulation, mainly by inhibiting lipogenesis. Data on polyunsaturated fatty acid (PUFA) supplementation are heterogeneous. Based on the available data, silymarin supplementation for the treatment of NAFLD seems to have a favourable effect.58 Antioxidant activity of carotenoids that protect against oxidative stress has been suggested also a possible mechanism to reduce metabolic syndrome. Vitamin A is inversely associated with body weight, BMI, waist circumference, and waist-hip ratio. There are synergistic and antagonistic interactions between vitamins A and D.59 There is no exact doses to be recommended in patients with sarcopenic obesity and NAFLD.
When administer vitamin D we need to be aware of zinc (Zn) which appears to cooperate in various of vitamin D functions, and might be also related to MS. Zinc enhances the activity of vitamin D dependent promoters.60 Zn status in individuals could be a very important factor when examining the association between vitamin D status and the risk of MetS. Treating of magnesium (Mg) deficiency is also very important in metabolic syndrome and obesity. It is also known that Mg deficiency is associated with a reduction in the active form of vitamin D and causes resistance to pharmacological doses of vitamin D.61 Recent research advise adding Vitamin K2 when treating Vitamin D deficiency. Vitamin K2 supplementation reduces the progression of carotid intima media thickness.62 A very recent study found that Vitamin D, K and Ca co-supplementation for 12weeks among diabetic patients with CHD had beneficial effects on carotid intima media thickness and metabolic status (reduction of HOMA-IR, hsCRP, cholesterol).63 By our opinion further studies are needed to elucidate the nutraceutical interventions in sarcopenic obese patients with fatty liver disease. Future research should aim to explore the mechanisms of accelerate aging in patients with NAFLD and the effect of complex pharmaceutical and nutraceutical interventions in patients with sarcopenic obesity and NAFLD.
Author contributions
MN, MP and VK equally contributed to this paper with conception, literature review, drafting, critical revision and editing. MB and AP contributed to the revision, approval and copyediting of the final draft.
Take home message
Obesity is a complex condition with increasing prevalence over the world. Visceral obesity is a subphenotype characterised by accumulation of metabolically active fat tissue. It is associated with variety of metabolic diseases generally leading to increased mortality and morbidity. Sarcopenia is a term used for normal decline of muscle tissue over the normal ageing. Obese and overweight persons are prone to develop early sarcopenia, related to sedentary lifestyle, nutritional deficiencies and insulin resistance. Sarcopenic obesity has been linked to obesity and consequently to features of metabolic syndrome like fatty liver disease. Complex and multidisciplinary approach is needed to diagnose, evaluate and treat vitamin D and other deficiencies in sarcopenic obese people with NAFLD.
None.
No potential conflicts of interest. No financial support.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.