eISSN: 2373-6372


Biliary cast syndrome (BCS) is an unusual complication of orthotopic liver transplantation (OLT). BCS is characterized by multiple intrahepatic biliary strictures, ductal dilatation, intrahepatic abscesses, and biliary anastomotic leakage. The symptoms of BCS usually include high fever, jaundice and cholestatic liver enzyme elevation; similar to the symptoms observed in some patients with intrahepatic bile duct stones. The etiology of cast development is not fully known, and its management is difficult. Limited success using endoscopic retrograde cholangiopancreatography (ERCP) or open exploration to clear casts has been reported, and failure usually results in retransplantation. We describe a diabetic man who developed a BC after deceased donor liver transplantation. Percutaneous transhepatic biliary cholangiography was performed and revealed a filling defect in both the right and left intrahepatic systems, extending down to the anastomosis. Percutaneous transhepatic choledochoscopy proved that it was consistent with BCs, and the casts were removed by stone retrieval basket, foreign body pliers, and adsorber.
ERCP, endoscopic retrograde cholangiopancreatography; OLT, orthotopic liver transplantation; BCS, biliary cast syndrome; MRCP, magnetic resonance cholangiopancreatography
A 48-year-old male hepatitis B carrier underwent deceased donor liver transplantation in China for cirrhosis and hepatocellular carcinoma two years ago. His immunosuppressive regimen included corticosteroid and tacrolimus. Immunoglobulin was administered for control of hepatitis B. In view of the persistent liver dysfunction, xanthochromia and skin itching one month ago, endoscopic balloon dilatation was performed and cholangiography showed multiple intrahepatic bile duct strictures. The patient transferred to us for further management of the strictures.
On admission, routine laboratory evaluation showed 67mmol/L total bilirubin (reference range , 3-22mmol/L), 12mmol/L conjugated bilirubin (reference range , 0-5mmol/L), 155U/L alanine aminotransferase (reference range , 0-40U/L), 129U/L aspartate aminotransferase (reference range , 0-40U/L), 1038U/L alkaline phosphatase (reference range , 38-126U/L), 884U/L g-glutamyl transferase (reference range , 6-35U/L) and 5.98´109cells/L white blood cell count with 14.6s prothrombin time (reference range , 9-14s).
Abdominal ultrasound and computed tomography demonstrated mildly dilated intrahepatic bile duct, normal common bile duct diameter, and normal Doppler flow. Magnetic resonance cholangiopancreatography (MRCP) showed T1 hyperintense and T2 hypointense linear filling defects in the right and left hepatic ducts, extending down to the anastomosis. Cholangiography showed multiple linear filing defects in the proximal hepatic duct proximal to the anastomosis, with extension into the intrahepatic system.
Endoscopic procedure
In the digital subtraction arteriography suite, with the patient placed in the supine position and under monitored care, percutaneous transhepatic biliary cholangiography was initially performed using a 22-gauge Chiba needle to inject the contrast medium from the right intercostal approach. The puncture site for percutaneous transhepatic biliary drainage was chosen depending on biliary anatomy, position of the BC, and the possibility of puncturing a dilated intrahepatic bile duct under ultrasound. Catheterization of intrahepatic bile ducts was performed in standard fashion. The metal wick-in-needle was removed and a hydrophilic wire was advanced through the metal mandrin into the dilated intrahepatic bile duct. An 8.5-F polyethylene biliary drainage catheter was inserted under the guide of the hydrophilic wire for one week of drainage. Cholangiography revealed a filling defect in both the right and left intrahepatic systems, extending down to the anastomosis, and it was consistent with a BC.
One week after the drainage procedures, the 8.5-F catheter was replaced with a 10-F catheter, with all side holes in the intrahepatic bile duct. The 12-F, 14-F and 16-F catheters were used in turn once weekly. Nutrition supplementation should be given to avoid protein malnutrition and electrolyte depletion. All drainage procedures were performed with the administration of broad-spectrum antibiotics. The microfiber choledochoscope was passed into the fistulous tract and observed the entire intrahepatic bile duct down to the common bile duct. A stone retrieval basket, foreign body pliers, and adsorber were used to remove the cast until it was not seen under the choledochoscope. Oral 750mg/d tauro-ursodesoxycholic acid was used to prevent cholestasis. Follow-up cholangiography revealed no residual filling defects, and the biliary tree was without obvious filling defect. The patient tolerated the procedure well without complications. In the two years after initial removal of the cast, the patient has remained asymptomatic.
BCS has an incidence of 2.5-18% after OLT.1 However, there are limited reports of BC in the non-transplant situation.2–4 Physically and morphologically, a BC appears as a dark, hardened material in the shape of the bile ducts, within the biliary ductal system. Its clinical symptoms usually include high fever, jaundice and cholestatic liver enzyme elevation. Potential consequences include cholangitis and graft damage or loss. Twenty-two percent of the BCS patients require repeat OLT.1 However, its mechanism of formation is uncertain. Some of the proposed etiological mechanisms include acute rejection, bile duct damage and ischemia, prolonged cold ischemia time, hemolysis, cholangitis, use of postoperative biliary drainage tubes, biliary infection, fasting, infection, biliary obstruction, supersaturation with cholesterol, mucosal damage, parenteral nutrition, abdominal surgery, head injury/prolonged intensive care, and parenteral nutrition.5–12
ERCP, percutaneous transhepatic cholangiography and MRCP are the main diagnostic methods of BCS, and multiple linear filling defects can be seen in the intrahepatic duct system.13–15 However, management of BCS is difficult. In the early days, surgical biliary reconstruction was the only available option for treating BCS.16 Other treatments such as endoscopic nasobiliary drainage, percutaneous transhepatic drainage, or retransplantation should be considered when complete removal is not feasible or the condition of the recipient deteriorates. Endoscopic cast extraction by ERCP has been described in many reports. However, this method has been usually used in extrahepatic BCS, and management of intrahepatic BCS is difficult, and failure usually results in retransplantation.17,18
BCs can prevent bile drainage, resulting in biliary obstruction and biliary tract infection. Percutaneous transhepatic cholangiography drainage is a common method for drainage of infected bile. After fistula extension, percutaneous transhepatic choledochoscopy can be implemented. We can observe the condition of the intrahepatic and extrahepatic bile ducts and remove multiple BCs with the help of choledochoscope, which is difficult by the conventional methods.19 In the process of removing BCs, we found that the BCs were shaped like the bile ducts, appearing as a hardened, dark material in the biliary ductal system with serious intrahepatic and extrahepatic bile duct injury. Some parts of the biliary mucosa showed patchy or strip defects, and mucosa ulcers were visible sometimes. After completely removing the BCs, the visualization of intrahepatic and extrahepatic bile ducts were improved, and patchy and bile duct mucosa were improved to varying degrees, showing reddish, smooth, rounded lumen and fresh bile secretion, and cholangiography displayed a very clear biliary tree (Figures 1–5).
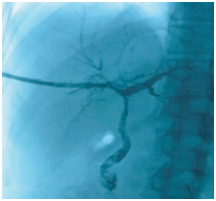
Figure 1 Cholangiography before BC removal. The intrahepatic bile ducts were poorly visualized, and patchy and tree-like negative shadows were seen.
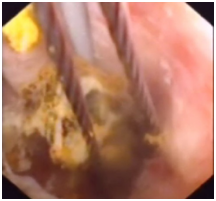
Figure 2 BC removal by choledochoscopy. The figure shows biliary mucosa swelling, erosion and ulceration, and the BC inside was caught by a stone basket.

Figure 3 BCs removed by percutaneous transhepatic choledochoscopy. Morphologically, BCs are shaped like bile ducts, appearing as hardened, dark material in the biliary ductal system.
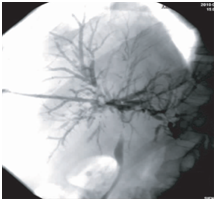
Figure 4 Cholangiography after BC removal. The first cholangiographic findings of the same patient after endoscopic therapy. Visualization of intrahepatic and extrahepatic bile ducts was improved, and patchy and tree-like negative shadows partially vanished.
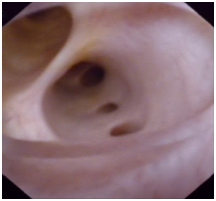
Figure 5 Choledochoscopic investigation after BC removal. The figure shows reddish, smooth, rounded lumen and fresh bile secretion.
There are many considerations for percutaneous transhepatic choledochoscopy. For different location of the BC, the appropriate puncture site should be selected. If the BC is in the right hepatic duct, we should puncture the left bile duct. This can reduce the difficulty of choledochoscopy and avoid the angle between the biliary duct and the choledochoscope. Choledochoscopic techniques should be used to reduce the incidence of bile duct mucosal damage and bleeding. BCs are often combined with bile duct stricture, located at the proximal end of the cast. Before removing the BC, bile duct stricture should be resolved.19 Selective endoscopic biliary visualization can be used to find occult BCS, and balloon dilatation or endoscopic high frequency electric cutting can resolve the biliary duct stricture.15,20
Percutaneous transhepatic choledochoscopy not only has a diagnostic role, but also can achieve the desired therapeutic effect with minimal invasion, repetition, and patient tolerance. With the popularity of endoscopic techniques and their improved operation by clinical physicians, an increasing number of patients with biliary complications after liver transplantation will benefit from this technique.
None.
Author declares there are no conflicts of interest.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.