eISSN: 2577-8307


Research Article Volume 2 Issue 6
1Ferdowsi University of Mashhad, Iran
2University of Sari Agricultural Sciences and Natural Resources, Iran
Correspondence: , Tel 98 913 308 8130,
Received: December 12, 2017 | Published: December 19, 2018
Citation: Ahani H, Tabatabaei SA. Impact of irrigation with saline water on morphology of sea buckthorn seedlings in nursery. Forest Res Eng Int J. 2018;2(6):326-333. DOI: 10.15406/freij.2018.02.00068
The experiment for evaluate the effect of salinity stress on the morphological behaviors of Elaeagnus rhamnoides (L.) A. Nelson seedlings were done in Torogh nursery in Iran country. This species were conducted based on completely randomized design in summer season. Non-stress (EC 0.8 dSm-1) and salt treatment (EC 12.03dS m-1) were used every two days. Several morphological traits of seedlings stressed-including height, diameter, leaf number, leaf area, shoot and root length, freshly and number of root, fresh and dry weight of stem, root and leaf and ratio of root to shoot, leaf to shoot, leaf area, specific leaf area, leaf weight and slenderness index were determined analyzed. Results indicated that diameter growth, end of period height, height mean and leaf area growth were significantly different at p<0.05 level and height growth and number of leaves reduced significantly at p<0.01 in comparison with control treatment. Analysis of variance for nonlinear regression in salinity stress indicated in the height and diameter significant differences of p<0.01 and leaf number not significant differences. Principal component analysis showed that in first axis (PC1) in salinity treatment dry weights leaf, stem and root, leaf area, root to shoot ratio, freshly, leaf area ratio decrease and were the most correlated with first axis. Thus, not recommend that to be done irrigation with EC 12.03 dS m-1.
Keywords: Hippophae rhamnoides, Iran, medicine, stress
Sea Buckthorn (Hippophae rhamnoides L.) from Elaeagnaceae family has become a crop of interest for the food processing industry. Accepted name in the plant list org of this species is Elaeagnus rhamnoides (L.) A. Nelson. The exact number of species in the genus Hippophae is still unclear however, there are considered to be seven species and Hippophae rhamnoides has nine subspecies.1 Hippophae rhamnoides, also known as common sea buckthorn is a species of flowering plant, native to the cold-temperate regions of Europe and Asia. It is a spiny deciduous shrub. The plant is used in the cosmetic industry, in traditional medicine (useful for the treatment of skin disorders resulting from bed confinement, stomach and duodenal ulcers cardiovascular diseases and perhaps growth of some tumors), as animal fodder and for ecological purposes. The plants have a very developed and extensive root system, and the roots live in symbiosis with nitrogen fixing Frankia bacteria. The roots also transform insoluble organic and mineral matters from the soil into more soluble states.2Vegetative reproduction of the plants occurs rapidly via root suckers.3 E. rhamnoides has a strong ability to maintain leaf water and can increase chlorophyll content, reduce the photosynthesis and water relations during drought stress.4,5 Seed germination at its lowest point of origin China with 32% and the most was in East Azerbaijan with 95%.6 Means of germination percent in seed pretreatments (control, cold, ice water, hot water, lime juice and Gibberellin acid) were 7.5, 23.75, 21.25, 0, 15, and 42.5 in field.7 3.75, 43.75, 17.5, 1.25, 15 and 37.5 in greenhouse8 and in laboratory were 33, 12, 41, 4, 9 and 32, respectively.9,10
Salinity stress is of the most serious abiotic stress factors causing environmental problems and limiting growth and crop productivity.11 Salt stress affects plant morphology and physiology; both whole plant as well as cellular levels, through osmotic and ionic stress. It disrupts plant water relations, resulting in a physiological drought or osmotic stress.12,13 Plants are often exposed to environmental stresses through a life cycle. Water stress caused by high salinity, drought or both, is one of the serious factors to limit plant productivity.14
Green plants are some of the most specious eukaryotes on terrestrial earth after insects and nematodes, with some 500,000 species and probably diversified from single land colonization. However, terrestrial plants are subject to often extreme and hostile changes in their environment, but, in contrast to many animals and prokaryotes, as sessile organism they are essentially unable to move away from rapidly changing adverse environmental conditions. Therefore, they have evolved a remarkable ability to cope with highly variable environmental stresses, including drought, cold and soil with changing salt and nutrient concentration, as part of their successful colonization of the ever changing terrestrial habitats.15 Saline water could affect some physical and chemical properties of soils, witch these properties can affect the soil as an ideal environment for plant growth.16 Salinity stress is a widespread environmental problem. Although considerable effort has been devoted to solve this problem, two very important aspects have been neglected, i.e. complex salt stress. 17Plants grown under field conditions are often subjected to a number of different stressors simultaneously, such as salt stress, high irradiance, drought stress, heat stress and potassium (K) deficiency.18
Plant growth and physiological and biochemical attributes was seriously affected due to salinity.19 Salinity affects the growth of the root and causes production of toxic ion effects on the plant growth and development and this toxicity reduce the shoot growth.20 Sharma et al.,21 & Shallan22 reported great reductions (50%) in growth of seedlings of a number of forest tree species due to salinity. Salinity causes high concentration of K and low concentration of Na in and activity of K+ and Na+ transporters and H+ pumps that generate the driving force for transport.23 Salt stress causes an initial water-deficit and ion-specific stresses resulting from changes in K+/Na+ ratios. Thus, it leads to an increased Na+ and Cl– concentrations that decrease plant growth and productivity by disrupting physiological processes, especially photosynthesis.24 Under saline conditions, the presence of excessive concentrations of Na+, Ca2+, Mg2+, Cl–, and SO42– in the external media may reduce nutrient availability, inhibit nutrient uptake and saturate binding sites. Competition and interactions between No3- and Cl-, Ca2+ and Na+, and Na+ and K+ in the substrates as well as within the plant frequently lead to ion imbalances that may result in nutrient deficiencies or ion toxicities.25 Plants have improved complex mechanisms systems for adaptation to salinity stress; these include the accumulation of osmoprotectants and the up-regulation of stress responsive pathways driven by specific transpiration factors.26,27
Leaf senescence is also correlated with increased membrane permeability at high salt concentration.28 Increasing salinity leads to a reduction and/or delay in germination of plants and death.29 Moreover, salt stress affects a wide variety of physiological and metabolic processes in plants in their vegetative stages leading to growth reduction and often reduces shoot growth more than root growth.30,31 Seedlings adapt to environmental stress by different mechanisms, including changes in morphological, physiological and biochemical processes.32
Objective
The aim of present study was to analyze the effects of salt stress on some morphological attributes of Sea buckthorn (Elaeagnus rhamnoides) (L.) A. Nelson plant.
Plant materials and salt–stress treatments
In this study the effect of salinity stress on morphological characteristics Elaeagnus rhamnoides species that seed from accessions were used in Qazvin origin of Iran to produce the mother plants. The four months old seedlings were grown in a natural environment for the rest of the experiment in the field of Torogh nursery of Mashhad city (the length of 59°38’00’’ geographical Eastern and 36°16’00’’ north latitude, altitude 990 meters). Seedlings after transfer to pots containing sandy loam, up to two month in terms of the usual nursery of irrigation were in well water treatment (every two days). Then physical experiments for determining relative particle (percentage of silt, sand and clay) were performed using the hydrometer Baykas on particles smaller than two mm. Rezaipoorbaghedar et al.,33 due to soil laboratory experiment of Research Center of Khorasan Razavi province (Table 1).
Soil properties |
pH |
EC |
Organic matter |
Sand |
Silt |
Clay |
Soil texture |
P |
K+ |
Moisture |
bulk density |
Value |
7.4 |
6.61 |
1.04 |
67 |
23 |
10 |
Sandy loam |
54.8 |
353 |
9.8 |
1.42 |
Table 1 Physical and chemical analysis of soils
From early summer treatments was conducted on E. rhamnoides seedlings to the salinity conditions (water source of KavirPark-Civil Gonabad with EC 12.03 dSm-1 in Table 2 and non-stress (were watered to 100% of field capacity by supplying an amount of water by weighting whole pot of each treatment equal to transpiration losses every other day, each time 150 ml day-1.). Plant sensitivity to salinity continually changes during the growing season. This study was conducted in a completely randomized design with twenty replicates during the summer.
pH |
E.Cw (dS m-1) |
Soluble cations (me l-1) |
Soluble anions (me l-1) |
||||||||
7.6 |
12.03 |
Ca+2+Mg+2 |
Ca+2 |
Mg+2 |
Na+ |
K+ |
HCO3-+CO3-2 |
CO3-2 |
HCO3- |
Cl- |
So4-2 |
20 |
10.7 |
9.3 |
101.5 |
0 |
3.7 |
Non |
3.7 |
50 |
67 |
||
Table 2 Chemical analysis of saline water used for irrigation
Plant growth and analysis
Stem growth characteristics were every 10 days monitored by measuring length (from substrate surface to the apical meristem), diameter (at the base) and by counting the number of leaves and a leaf area per month, so that the base of each treatment. 5 selection seedling and each seedling 5 leaf selection of basic randomized to using Auto CAD software in meters squared size was measured (Figure 1). Three plants were removed from the pots at the end of the experiment in early fall and divided into the aboveground part (leaves and stem) and belowground part (roots), then the fresh weight were taken at electrical balance in grams. Biomass samples were then placed in an oven run at (70°C, 48 h) up to constant weight.34–36 These dried plants were weighed to record the plant dry mass.
After the plants were cut and separated into different parts, the samples were obtained for morphological analyses.
Root to shoot ratio (R/S):37 Leaves to shoot ratio (L/S):38
Leaf area ratio (LAR) an index of the amount of spilled leaves the plant: ; Specific leaf area (SLA) is a measure of leaves: ; Leaf weight ratio (LWR) and freshly:39 ;and ; Slenderness40
Statistical analysis and processing
The experiment was arranged in the base of completely randomized design. The normality tested by (Kolmogorov-Smirnov) using SPSS version 19, data collected were subjected to correlation between various parameters and t-independent comparison of characteristics between the two measurements was used for stress. Pearson correlation coefficients, replication analysis measurements of height, diameter and leaf number in six and leaf area in two times calculated with four different multivariate tests (Wilks' Lambda, Pillai's Trace, Hotelling-Lawley Trace and Roy's Greatest Root) and ANOVA of nonlinear regression using the statistical software SAS version of the 9.1. Using PC-ORD software version 4.17 to principal component analysis (PCA). Diagrams were drawn using the Excel software for growth average height, diameter, leaf numbers and leaf area was conducted by stress condition initiation until end of the experiment.
Height and number of leaves trait of the end of the season, height and number of leaves mean and changes in the total number of green leaves for salinity stress conditions are not correlated but in non-stress showed a positive correlation with the diameter mean at one percent level. Diameter end of the season in two treatments showed a positive correlation with the diameter mean at one percent level. Slenderness index average and end of the growth slenderness index for control at five percent level but in salinity stress is not correlated (Table 3). h6: End of the period height, hm: Height mean, d6: End of the period diameter, (h/d) m: Slenderness index mean, (h/d) 6: End of the slenderness index, nl6: End of the period No. of leaves, nl: No. of leaves, nlm: No. of leaves mean. ns: Not significant, *p<0.05 and **p<0.01.
Treatment |
|
h6 |
hm |
d6 |
(h/d)m |
(h/d)6 |
nl6 |
nl |
nlm |
Control |
Diameter mean |
0.86** |
0.86** |
0.99** |
0.52* |
0.49* |
0.88** |
0.82** |
0.81** |
Salinity |
0.34ns |
0.36ns |
0.99** |
-0.21ns |
-0.22ns |
0.35ns |
-0.05ns |
0.39ns |
Table 3 Correlation coefficients among some morphological traits with diameter mean under two treatments
Results of the t-independent test comparing the two treatments (control-salinity) was indicated that height growth and number of leaves at the one percent level and diameter growth, end of period height, height mean and leaf area growth were significant at the five percent level and other traits not significant (Table 4). Repeated measures analysis of such statistics, Wilks' Lambda, Pillai's Trace, Hotelling-Lawley Trace and Roy's Greatest Root of the time multiplied by treatment morphology traits of the measures indicated that the diameter of the control-salinity treatment there is no significant difference but in height, leaf number and leaf area showed significant differences at the five percent level (Table 5).
Morphology traits |
Control mean |
Salinity mean |
Mean difference |
t-value |
Diameter growth |
0.12±0.02 |
0.06±0.01 |
0.06±0.02 |
2.08* |
End of period diameter |
1.34±0.10 |
1.19±0.06 |
0.14±0.12 |
1.21ns |
Diameter mean |
1.28±0.09 |
1.16±0.06 |
0.13±0.11 |
1.12ns |
Height growth |
9.35±1.38 |
3.45±0.49 |
5.90±1.46 |
4.03** |
End of period height |
19.22±2.24 |
13.20±1.14 |
6.02±2.51 |
2.39* |
Height mean |
18.94±2.24 |
13.07±1.14 |
5.86±2.52 |
2.33* |
No. of leaves |
17.75±3.53 |
4.95±1.95 |
12.80±4.04 |
3.17** |
End of period no. of leaves |
45.90±7.57 |
29.30±3.38 |
16.60±8.29 |
2.00 ns |
Mean no. of leaves |
37.47±6.11 |
29.05±3.28 |
8.42±6.94 |
1.21 ns |
Leaf area growth |
4.26×10-3±1.66×10-3 |
-4.92×10-4±4.59×10-4 |
4.75×10-3±1.72×10-3 |
2.76* |
End of period leaf area |
1.11×10-2±3.31×10-3 |
4.66×10-3±1.21×10-3 |
6.44×10-3±3.52×10-3 |
1.82ns |
Leaf area mean |
8.96×10-3±2.66×10-3 |
4.91×10-3±1.28×10-3 |
4.06×10-3±2.95×10-3 |
1.37ns |
Table 4 Independent samples test comparison; Mean±SE
Ns, not significant; *p<0.05 and **p<0.01.
Morphological traits |
Wilks' Lambda |
Pillai's Trace |
Hotelling-Lawley Trace |
Roy's Greatest Root |
Height |
0.64 |
0.35 |
0.55 |
0.55 |
3.07* |
3.07* |
3.07* |
3.07* |
|
Diameter |
0.77 |
0.23 |
0.29 |
0.29 |
1.61ns |
1.61ns |
1.61ns |
1.61ns |
|
No. of leaves |
0.67 |
0.32 |
0.48 |
0.48 |
2.65* |
2.65* |
2.65* |
2.65* |
|
Leaf area |
0.46 |
0.54 |
1.17 |
1.17 |
9.39* |
9.39* |
9.39* |
9.39* |
Table 5 Effect treatment×time morphological traits control-salinity treatment
Ns, Not significant;* p<0.05; The number of second rows for each term showed statistics equivalent F
As it has been shown is in Table 6 the final nonlinear regression model, the coefficient of determination, correlation coefficient, standard deviation error and variance inflation factor without stress and salinity stresses treatments for morphological traits characters of height, diameter and leaf number. Analysis of variance for nonlinear regression in measurement two months in seven times indicated that height and diameter trait in control and salinity stress significant differences of one percent. In leaf number trait showed in control significant differences of one percent but in salinity stress not significant differences (Table 7).
Morphological traits |
Treatment |
Regression |
R2 |
R |
Coef var |
Std. error |
VIF |
Height |
Control |
y =-0.3551x2 +4.4467x -4.4929 |
98.17% |
0.99 |
38.96 |
1.14 |
1.00 |
Salinity |
y =-0.125x2 +1.5214x -1.3643 |
95.21% |
0.96 |
32.81 |
0.35 |
1.00 |
|
Diameter |
Control |
y =-0.0029x2 +0.0446x-0.0493 |
96.67% |
0.98 |
39.62 |
0.02 |
1.00 |
Salinity |
y =0.0007x2+0.003x+0.0011 |
92.07% |
0.95 |
53.85 |
0.01 |
1.00 |
|
No. of leaves |
Control |
y =-0.2702x2 +5.3298x -5.6929 |
94.04% |
0.96 |
50.79 |
2.47 |
1.00 |
Salinity |
y =-0.2702x2 +2.7476x -1.5214 |
79.04% |
0.89 |
7.55 |
0.14 |
1.00 |
Table 6 Nonlinear regression model growth for morphological traits
X, Measurement time; y, Height; diameter and No. of leaves traits and Variance Inflation Factor (VIF).
Morphological traits |
Treatment |
Mean Square |
F-value |
Height |
Control |
72.24 |
29.77** |
Salinity |
7.61 |
21.61** |
|
Diameter |
Control |
0.01 |
55.35** |
Salinity |
0.002 |
46.35** |
|
No. of leaves |
Control |
280.98 |
57.76** |
|
Salinity |
9.6 |
4.66 ns |
Table 7 ANOVA results of nonlinear regression model growth for morphological traits
Ns, Not significant; **p<0.01.
Polynomial growth curve with a slope showed, reducing the impact of salinity on growth traits height, diameter, leaf number and leaf area (Figure 2). End of the growth period showed average height and diameter growth, leaf number and leaf area in salinity stress value of decrease. Height growth in salinity in 30th start day decrease significantly (F6= 5.40; p=0.0256) as compared with control treatment but the 40th, 50th and 60th day showed significant (F6= 6.08, p=0.0183; F6= 5.92, p=0.0197 and F6= 5.73, p=0.0217 respectively) and total height growth indicated decrease significantly (F6= 16.22, p=0.0003). In diameter and number of leaves showed that no significant beginning to end of the experiment between the two treatment groups, but in total diameter growth and number of leaves indicated decrease significantly (F6= 4.32, p=0.0445 and F6= 10.03, p=0.0030 respectively). Principal Components Analysis showed first and second axis in control and first axis in salinity stress, Eigen value treatments is greater than the statistics Broken-Stick a result of the first and second axis of variation accounted for a significant share of the mean and of the use is analysis and other axes are not significant (Table 8).
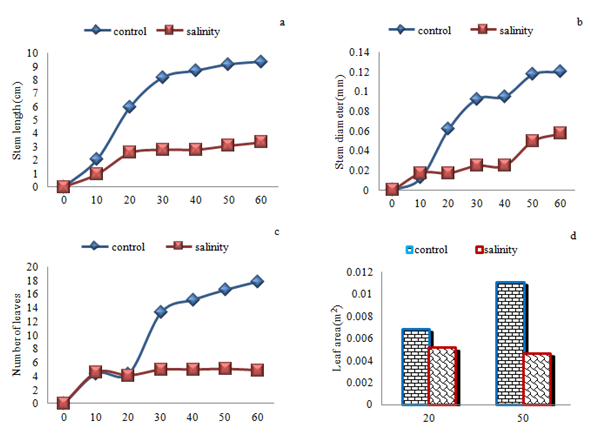
Figure 2 Average growth rates over time for stem length and diameter, leaf number and leaf area of (Elaeagnus rhamnoides (L.) A. Nelson) subjected to varying effect of salinity stress. Day 0 represents the first day the experimental treatments were applied.
Treatment |
Axis |
Eigen value |
Percent of Variance |
Broken-stick Eigen value |
Control |
1 |
8.795 |
79.957 |
3.02 |
2 |
2.205 |
20.043 |
2.02 |
|
Salinity |
1 |
9.987 |
90.793 |
3.02 |
|
2 |
1.013 |
9.207 |
2.02 |
Table 8 Variance extracted of control- salinity treatment
Variables that have the greatest equity without positive and negative symptoms are known as the most important variable that is correlated with the first axis and so are the next variables. According to Table 7 associated with non-stress the variables (dry weight leaf and stem, total dry weight, leaf area, leaf to shoot ratio, leaf area ratio, specific leaf area and leaf weight ratio) have most correlated with the first axis (PC1) as a result, the largest share of mining this axis and (dry weight root, root to shoot ratio, freshly) variables were the most correlated with the second axis (PC2). In salinity stress the variables (dry weights leaf, stem and root, leaf area, root to shoot ratio, freshly, leaf area ratio and leaf weight ratio) have most correlated with the first axis (PC1). Positive and negative symptoms value of special, to show the axis of extraction relationship between variable of interest positive and negative, respectively (Table 9).
Morphological trait |
Control |
|
Salinity |
|
|---|---|---|---|---|
|
PC1 |
PC2 |
PC1 |
PC2 |
dry weight leaf |
0.3299* |
0.1396 |
-0.3153* |
0.0831 |
dry weight stem |
-0.3371* |
-0.0110 |
-0.3164* |
0.0189 |
dry weight root |
-0.2248 |
0.5020* |
-0.3040* |
0.2760 |
total dry weight |
0.3048* |
-0.2880 |
0.2668 |
-0.5343* |
leaf area |
-0.3365* |
0.0417 |
-0.3139* |
-0.1243 |
root to shoot ratio |
0.2605 |
0.4277* |
0.3070* |
0.2414 |
leaf to shoot ratio |
0.3372* |
0.0097 |
-0.2939 |
0.3684* |
freshly |
-0.0326 |
-0.6703* |
0.3141* |
0.1211 |
leaf area ratio |
-0.3359* |
-0.0593 |
-0.3049* |
-0.2661 |
specific leaf area |
-0.3352* |
-0.0738 |
-0.2574 |
-0.5782* |
leaf weight ratio |
0.3363* |
-0.0484 |
-0.3161* |
0.0449 |
Table 9 Principal Component Analysis coefficients of morphological trait
There is a one research from Ahani et al.,4,5 for drought tolerance of the species of sea buckthorn (Elaeagnus rhamnoides) had done khorasan province of Iran and observes that drought tolerance was highly to the physiology in E. rhamnoides. With the extension of drought stress from two to twelve days, E. rhamnoides seedlings water use efficiency was increased; between first and second treatments, also between third and fourth treatments was observed significant differences. Relative water content gradually was declined with decreasing water supplies. Water potential was decreased, while drought was increased from first to last treatment. Water saturation deficit gradually was increased by accelerating drought in all treatments. Water saturation deficit values did not differ significantly between treatments 3 and 4. Significant difference at 5% levels were not observed between 8 and 12 days irrigated in both of R and S weight, but in all treatments was decreased toward drought. The photosynthetic rate (a) was positively correlated with SPAD and DS (dry stem) but negatively correlated with the chlorophyll concentration index (CCI) and DR (dry root) in all of treatments except 12 day irrigation. CCI was positively correlated with DR, in 2 day treatment, with SPAD and DR in 8 day with all of traits in 4 day and 12 day but negatively correlate with others. There were significant negative correlations between SPAD with DR in first watering regime and DS in third watering regime. Between DR and DS were significant positive correlations only in 12 day treatment but significant negatively in 2 day and 8 day watering regime measured. We concluded SPAD and a was tolerated efficiently between 4day and 8day, then we can irrigate seedlings more than 4day and less than 8day treatment.
Growth reduction is generally observed in plant exposed to salinity stress. Therefore, saline water causes the decreasing water potential and increases transpiration plants with reduced morphological traits compared with the non-stress. The result in this research similar to Hokmabadi et al.,41 reported relative growth rate decreased with time for all treatments. Salt treatment significantly reduce relative growth rate, net assimilation on a leaf weight basis but not leaf weight ratio. Daneshvar & Kiani42 Data analyzed and results found that branches length in Elaeagnus cultivar (Annabi) was significantly less than other cultivars (Hoofi, Shami, Kalaie and Shekari) but the number of branches was not affected by salinity. Root, shoot and leaf dry weight were affected by salinity, cultivar and interaction between them. Finally Russion olive is appraised as a resistant species to salinity and it seems that species from dry areas are more tolerant. Daneshvar et al.,43 observed salinity did not significantly affect leaf and stem dry weight at 6 dS m-1 of salinity but over 6 dS m-1 the root dry weight decreased significantly. Although leaf number decreased significantly by increase in saline levels, but root dry weight and seedling's height have not been influenced by salinity, significantly. Niyaei fard et al.,44 in research vegetative and physiological responses of olive trees to antioxidants and salinity were found salinity causes the decreasing morphological growth such as dry weight shoot, shoot length, dry weight root and root to shoot ratio. Tabatabaei et al.,45 in Celtis caucasica Willd seedlings indicated that under salt stress (EC 12.03 dS m-1) height growth, number of leaves, leaf area at the end of growth period and leaf area growth were significantly different in comparison with control treatment. Results of repeated measures analysis show that effect of time salinity interaction for diameter was not significant but for height and leaf area had significant difference. Shariat & Assareh46 in research on the seedlings of eight Eucalyptus species viz. E. kingsmillii, E. tetragona, E. salubris, E. occidentali, E. microtheca, E. camaldulensis, E. globules and E. sargentii under salt solution (0, 50, 100, 150 and 200 mM of NaCl) were found Eucalyptus sargentii as the most tolerant species had the optimum growth up to 200 mM NaCl. Salinity affects shoot growth and leaf development;47 Also causes the water stress in the plant which was responsible for limited growth in root and shoots length. Low level salts concentration did not affect the plant but by increasing its levels it has harmful effects.48
Salt stress is known to be one of the most important abiotic stresses and seriously affects survival. The deleterious effects of excessive salinity on plant growth are associated with (1) low osmotic potential of soil solution (water stress), which reduced and availability of water to plants, (2) nutritional imbalance effect on specific ionic (salt stress) and (4) a combination of all the 3 factors.49,50 All of these cause adverse pleiotropic effects on plant growth. Growth inhabitation is the primary injury that leads to other symptoms although programmed cell death may also occur under severe salinity shock. Salt stress induces the synthesis of abscisic acid which closes stomata when transported to guard cells. As a result of stomata closures, photosynthesis declines and photoinhibitation and oxidative stress occur. An immediate effect of osmotic stress on plants growth is its inhabitation of cell expansion either directly or indirectly through abscisic acid.51 Nutrient disturbances under salinity reduce plant growth by affecting the availability, transport and partitioning of nutrient. However, salinity can differentially affect the mineral nutrition of plants. Under saline conditions, a reduce plant growth due to specific ion toxicities (e.g. Cl- and Na+) and ionic imbalances acting on biophysical and metabolic components of plant growth occur.52
During stress condition plants need to maintain internal water potential below that of soil and maintain turgor and water uptake for growth.53 This requires an increase in osmotic, either by uptake of soil solutes or by synthesis of metabolic solutes. Decrease in water conductance of the plasma membrane causing reduction in plant height.54 Salinity stress reduces the ability of plants to take up water and this causes reduction in growth rate along with a suite of metabolic changes similar to those caused by water stress.55
Salt toxicity primarily occurs in the older leaves where Na and Cl build up in the transpiring leaves over a long period of time, resulting in high salt concentration and leaf death. Leaf injury and death is probably due to the high salt load in the leaf that exceeds the capacity of salt compartmentation in the vacuoles, causing salt to build up in the cytoplasm to toxic levels.56,57 If new leaves are produced at a rate greater than the rate at which old leaves die, there are enough photosynthesizing leaves for the plant to flower and produce seeds, although at reduced numbers.57 The reduction of leaf area could be attributed to the negative effect of stress on the rate of cell elongation, cell volume and cell number.58 Reduction in shoot growth due to salinity is commonly expressed by a reduced leaf area and stunted shoots. Final leaf size depends on both cells division and cell elongation. Leaf initiation, which is governed by cell division, was shown to be unaffected by salt stress in Beta vulgaris, but leaf extension was found to be a salt sensitive process.59 Thus, cell division in leaves of Beta vulgaris appears less salt sensitive than cell elongation. On the other hand, cell numbers in leaves were reduced by salinity.
Islamic Republic of Iran is located at the arid and semi-arid region of Asia. Thereupon, study and various tests associated with forest trees are essential to the water-economy, conservation and development of natural resources. The study showed that despite of decrease in morphological characteristics, this species has been able to tolerance this amount of salt and no died that showed comparative tolerance this species. Therefore offers for Elaeagnus rhamnoides species resistance threshold electrical conductivity (EC= 12.03dSm-1) of the lower levels used to determine which however need to do other tests because the morphological indicators studied here were not able to clearly explain the differences in salt tolerance and Physiology parameter need to test (i.e. Water Use Efficiency (WUE), Relative Water Content (RWC), Water Potential (WP), Water Saturation Deficit (WSD), Chlorophyll content and Photosynthetic of leaves).
This study was supported by the Sari University of Agricultural Sciences & Natural Resources and Natural Resources & Watershed Administration of the Khorasan Razavi province, Iran.
The author declares there are no conflicts of interest.

©2018 Ahani, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.