eISSN: 2577-8307


Research Article Volume 2 Issue 3
1Co-founder of the Lightfoot Institute, Canada
2Goodman Cancer Research Centre of McGill University, Canada
Correspondence: H Douglas Lightfoot, Co–founder of the Lightfoot Institute, 8 Watterson, Baie–D’Urfe, QC, H9X 3C2, Canada, Tel 514–457–5637
Received: May 25, 2018 | Published: June 28, 2018
Citation: Lightfoot HD, Orval AM. Carbon dioxide: sometimes it is a cooling gas, sometimes a warming gas. Forest Res Eng Int J. 2018;2(3):169-174. DOI: 10.15406/freij.2018.02.00043
The laws of physics, namely the gas laws, were applied to the gases in the atmosphere that act as ideal gases. The results show that as air temperature increases from winter to summer CO2 is a cooling gas and from summer to winter it is a warming gas regardless of its concentration in the atmosphere. This is contrary to the commonly held belief that CO2 always warms the atmosphere. Back radiation is the sum of the radiation of all of the greenhouse gases back to the Earth. It is a measured value and increases with temperature and vice versa. Back radiation acts opposite to that of CO2, methane and the trace gases. On average, the latter account for 1.2% of back radiation and water vapor accounts for 98.8%. The effect of CO2, methane and the trace gases on atmospheric temperature and climate change is so small as to be negligible.
Keywords: carbon dioxide, water vapor, back radiation, atmospheric temperature, climate change, radiative forcing
Current government policies are designed to reduce the consumption of fossil fuels because of the widespread belief that increasing levels of carbon dioxide (CO2) in the atmosphere will cause large and dangerous warming of the Earth’s atmosphere. Because 80% of the world’s energy comes from coal, oil and natural gas and are vital to providing the food, clothing and shelter that are the cornerstone of the well–being of our people, it is imperative that we examine the validity of this widespread belief.
The concept that carbon dioxide (CO2) is causing climate change comes from the fact that the concentration of CO2 is increasing in the atmosphere from the burning of fossil fuels. This has increased the warming effect of CO2 by approximately 1.7 Watts per square metre (Wm–2) since 1750.1 This is the basis for the belief that CO2 is increasing atmospheric temperature. This belief is reinforced by the IPCC. They suggest that doubling of CO2 concentration in the atmosphere from 280 ppmv in 1750 to 560 ppmv is expected to increase the atmospheric temperature by a dangerously high 3°C.
A key argument against CO2 as the cause of climate change uses the laws of physics, namely the gas laws, and the measured value of back radiation to show the effect of CO2 on atmospheric temperature. The gas laws applied to the gases in the atmosphere show CO2 is a warming or a cooling gas depending on the time of year regardless of its concentration. Radiation warming from CO2 is a small part of back radiation, which is the total radiation back to the Earth from all of the greenhouse gases. As shown in Figure 1, the portion of back radiation by CO2 is very small compared to the total, approximately 0.6%. Virtually all of back radiation comes from water vapour and it acts opposite to that of CO2. Thus, while CO2 is trying to warm the atmosphere, water vapor is cooling it and vice versa.
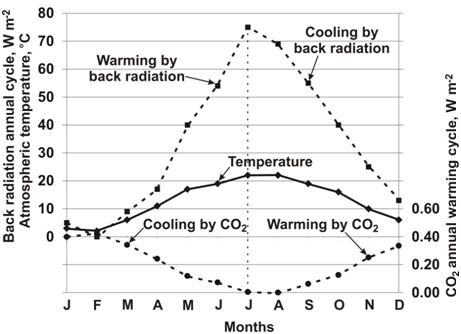
Figure 1 The warming effect of back radiation rises by 70Wm–2 from January to July while CO2 causes cooling of 0.45 Wm–2as its concentration falls.
The purpose of this paper is to provide the details for constructing Figure 1 and to provide the evidence that the warming effect of CO2 on the atmosphere is small enough to be negligible. This paper builds on the statement in Lightfoot et al.,2 “back radiation acts in opposition to the warming effect of the CO2” and provides new information. It provides the details necessary for the construction of Figure 1 that involve several scientific disciplines, such as, temperature and relative humidity records for Hamburg, Germany; the gas laws of Boyle and Charles/Gay–Lussac; psychrometric charts or programs; the back radiation measured at Hamburg; the concentration of CO2 measured at Mauna Loa and the relative warming effect of various greenhouse gases (GHG) from Figure SPM.5.1
The construction of Figure 1 leads to the evidence that all of the greenhouse gases can warm or cool the atmosphere depending on the conditions. Finally, evidence is provided showing the total warming or cooling effect of all of the ideal greenhouse gases, i.e., CO2, methane and the trace gases, is approximately 1.2% of that of water vapor.
The starting point of this paper is an explanation of the baseline concentration of CO2 as reported by the Mauna Loa Observatory. This baseline allows the calculation of the concentration of CO2 at any time or place on Earth using the gas laws.
It is known that the concentration of water vapour as a GHG at any location can change over relatively short time periods and can be affected by atmospheric temperature. Similarly, the concentration of CO2 and its warming effect can change depending on the elevation of the location and atmospheric temperature. In this paper, we show how to calculate the concentration of CO2 at any location on Earth where the elevation and temperature are known and including dilution by water vapor.
Carbon dioxide acts as an ideal gas under the conditions of temperature and pressure experienced on Earth and obeys the gas laws, i.e., the physical laws relating pressure, temperature and volume of a gas that were discovered by Boyle and Charles/Gay–Lussac. Significant differences in elevations, atmospheric temperature and relative humidity (RH) are the cause of significant differences in CO2 concentration around the Earth. These differences make it difficult to determine whether or not the average CO2 concentration is increasing or decreasing. Thus, it is necessary to establish a system of measurement that eliminates the effect of pressure, atmospheric temperature and RH variations on CO2 concentration and to establish a consistent baseline.
To develop a baseline, in 1959 Charles Keeling started measuring the mole fraction of CO2 in dry air in parts per million molecules of dry air3,4 at Mauna Loa and reported the results as parts per million by volume (ppmv) of dry air. The same measurements that Keeling started are continued today.5 There is some confusion about the number that is reported daily by the Mauna Loa Observatory. From the start of measurements in 1959 through the IPCC First6 and Third7 Assessment Reports the number was always reported as ppmv. Beginning with the IPCC Fourth Assessment Report,8 the number was defined as parts per million molecules of dry air (ppm). This definition is related to the composition of the atmosphere and composition does not change with pressure or temperature. It is also used to estimate the dilution of CO2 by water vapor. However, it says nothing about the concentration that determines the warming effect of CO2. Conveniently, the difference between the two values is very small, i.e., the ppmv value is approximately 0.3ppm lower than the ppm value at CO2 concentration of 400 ppm.9 Because the difference is very small, approximately 0.075%, the same value is useful for each definition, ppm and ppmv, with negligible error.
For the ppmv definition, the baseline conditions are the well–known Standard Temperature and Pressure (STP, 0 °C and atmospheric pressure, 101325 Pascals) in dry air. This is consistent with the practice of measuring all of the calibration gases at STP10 at the Mauna Loa Observatory to ensure the accuracy of the CO2 measurements. This STP baseline and the gas laws provide a means of calculating the CO2 concentration at any location on Earth where the temperature, RH and elevation (The Engineering Toolbox)11 are known. It is CO2 concentration that determines the actual warming, or greenhouse effect, of CO2 on the atmosphere, i.e., the number of CO2 molecules per cubic metre or ppmv. The difference in warming effect, or radiative forcing (RF), of CO2 between two concentrations is defined by the approximate logarithmic expressions developed in the TAR,1 such as the simplified ΔRF = αln(C/Co). The suggested value of α is 5.35. In AR4, the IPCC appears to have used a constant of 5.22 instead of 5.35 to calculate the difference between 275 and 378 ppmv as 1.66 Wm–2 in Figure SPM.2.8 The constant of 5.22 is used in this study.
The U.S. Department of Commerce, National Oceanic & Atmospheric Administration, NOAA Research website titled: How we measure background CO2 levels on Mauna Loa12 gives the composition of the atmosphere when the portion of CO2 is 372 ppm, and explains how to calculate the dilution of CO2 by water vapor in the following paragraph and Table 1:
A |
B |
C |
|---|---|---|
|
Dry air |
3% wet air |
Nitrogen |
780,900 |
757,473 |
Oxygen |
209,400 |
203,118 |
Water vapour |
0 |
30,000 |
Argon |
9300 |
9021 |
Carbon dioxide |
372 |
360.8 |
Neon |
18 |
17.5 |
Helium |
5 |
4.9 |
Methane |
2 |
2 |
Krypton |
1 |
1 |
Trace species (each <1) |
1 |
1 |
Total |
1,000,000 |
1,000,000 |
Table 1 Correction of CO2 concentration for dilution by water vapor.
“For example, 372 parts per million of CO2 (abbreviated as ppm) means that in every million molecules of (dry) air there are on average 372 CO2 molecules. The table below gives an example for 372 ppm CO2 in dry air. All species have been expressed as ppm, turning 78.09% nitrogen into 780,900 ppm. The rightmost column shows the composition of the same air after 3% water vapor has been added.” Using the method of Column C, at the current CO2 concentration level of approximately 400 ppmv, the dilution by 30,000 ppmv of water vapor, 3%, is 12 ppmv. Because of rounding, columns B and C in Table 1 actually add to 999,999.
Parts 1 and 2 of Table 2 are an example of a spreadsheet table constructed to calculate the concentration of CO2 at Hamburg, Germany as in columns J and K of Part 2. The procedure can be readily followed by reading the cell titles in alphabetical order. Table 2 can be adapted to calculate the concentration of CO2 at any place on Earth.
Average day |
A |
B |
C |
D |
E |
F |
July 1 |
4 PM |
22 |
58 |
1.0123 |
W |
0.009610 |
Jan 1 |
3 PM |
3 |
86 |
1.0123 |
W |
0.004047 |
Figure 2
Part 2 Spreadsheet table for calculating the concentration of CO2.
Average day |
G |
H |
I |
J |
K |
L |
July 1 |
9610 |
15450 |
407.9 |
377.1 |
371.3 |
41.6 |
Jan 1 |
4047 |
6507 |
407.9 |
403.1 |
400.5 |
16.2 |
Part 2 Spreadsheet table for calculating the concentration of CO2.
Column I of Part 2 is the baseline CO2 concentration of 407.9 ppmv issued on May 9, 2016 by the National Oceanic and Atmospheric Administration (NOAA) expressed as ppmv at STP. This value is above the average value for 2016 of approximately 404 ppmv. From the 407.9 baseline, the average July 1, concentration of CO2 of 377.1 ppmv in dry air is calculated using the gas laws to adjust for pressure (elevation) and temperature (degrees, K) as in Equation 1:
July 1 CO2 concentration = 407.9 x (101230/101325) x (273/295) = 377.1 ppmv in dry air (1)
The method of Column C in Table 1 is used to calculate 371.3 ppmv of CO2 after dilution by 15,540 ppmv of water vapour, Column H.
Similarly, the January concentration of CO2 is calculated as in Equation 2:
January 1 CO2 concentration = 407.9 x (101230/101325) x (273/276) = 403.1 ppmv in dry air (2)
Regardless of the concentration of CO2 in the atmosphere, the gas laws always show a decrease in concentration with increased temperature and vice versa.
Note that Column F in Part 1 requires the MegaWatSoft psychrometrics program (MegaWatSoft)13 to be installed behind an Excel spreadsheet. The same result can be obtained using physical psychrometric charts available from the American Society of Heating, Refrigerating and Air–Conditioning Engineers, Inc. (ASHRAE).14
The values in Column H are calculated from Column F for July as follows:
(0.009610 x 1,000,000) = 9610 ppm x (28.9645/18.016) = 15,450 ppmv (3)
where 28.9645 is the molecular weight of air and 18.016 is the molecular weight of water vapor.
The ratio of the number of water molecules to the number of CO2 molecules in Column L is calculated using the baseline concentration of 407.9 ppmv. The ratio of the number of molecules is proportional to the concentration in parts per million by volume, ppmv. It is shown to help the reader better visualize the large differences in concentrations and warming effects between water vapor and CO2.
A table similar to that of Table 2 was used to construct Figure 2, which is a monthly plot of the sun angle, average atmospheric temperature at Hamburg, CO2 concentration in dry air and water vapor concentration (H2O/CO2 ratio) on the same graph. The H2O/CO2 ratio is the ratio of the number of water molecules to CO2 molecules.
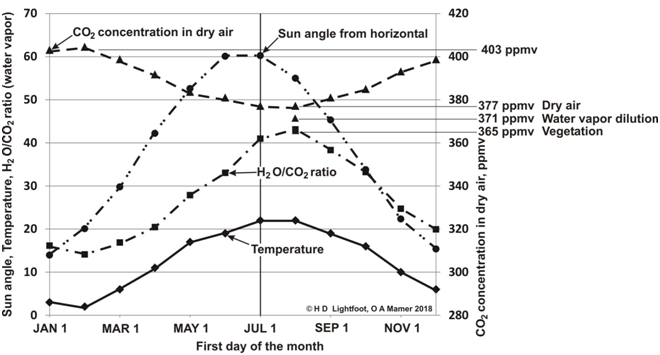
Figure 2 Seasonal record of sun angle, atmospheric temperature, CO2 concentration in dry air and water vapor concentration (H2O/CO2 ratio) for Hamburg, Germany.
In Figure 2, it is the gas laws that cause CO2 concentration in dry air to fall by 26 ppmv from 403 to 377 ppmv from January to August. As the air warms it expands and there are fewer molecules of CO2 per cubic metre. Water vapor dilution causes CO2 concentration to drop another 6 ppmv and the effect of vegetation in the Northern Hemisphere drops it another 6 ppmv to 365 ppmv. Over the same time period, the temperature rises from 3°C to 22°C. This is clear evidence that increased atmospheric temperature is associated with reduced CO2 concentration.
In contrast to CO2 concentration, actual weather records show water vapor concentration, the H2O/CO2 ratio, moves in synchronization with atmospheric temperature. For example, Table 2, Parts 1 and 2, show from January 1 to July 1 at Hamburg the temperature rises 19°C (Col. B), CO2 concentration falls 26 ppmv (Col. J), water vapour rises by 9033 ppmv (Col. H), and the H2O/CO2 ratio raises 25.4 units (Col. L).
This is conclusive evidence that from winter to summer the warming by water vapor counteracts the small cooling by CO2. Conversely, from summer to winter, the warming effect of CO2 tends to warm the air as water vapor is cooling it. But the effects by CO2 each time are so small as to be negligible. This evidence comes to light because the gas laws show that in the atmosphere CO2 concentration falls as temperature rises. By examining the warming curves for water vapor2 and CO2 Lightfoot et al.,15 versus increased concentration separately from the atmosphere it appears logical to assume they are additive. Climate models consistently project increased atmospheric warming with increased CO2 concentration in the atmosphere. This could not happen if the models accounted for the fact that the large warming and cooling effects of water vapor counteract the small cooling and warming effects of CO2.
The inverse relationship between CO2 and temperature also occurs daily as shown in Figure 3 for Montreal, Canada. For example on an average July 1 increasing temperature from 17 to 24°C towards the afternoon decreases the CO2 concentration from 383.7 to 374.7 ppmv, a difference of 9 ppmv. Similarly to Figure 2, the plot of daily records also shows the H2O/CO2 ratio moves in the same direction as the temperature.
Figure 1 shows the measured difference in back radiation between January and July at Hamburg on the left hand axis as it increases from 298 to 368 Watts per square metre (Wm–2). Over the same time period, the right hand axis shows the ΔRF of CO2 calculated by ΔRF = 5.22ln(C/Co) falls by 0.40 Wm–2, or 0.6% of that of back radiation. The key point is that from winter to summer the warming by back radiation works against the small cooling effect of CO2 when the two are together in the atmosphere. Similarly, from summer to winter, the cooling effect of water vapor works against the small warming effect of CO2.
Table 3 summarizes the results of applying the calculations of Table 2 to the average weather records at four locations from the Arctic to the Tropics. These locations with latitude and elevation are Barrow, Alaska (71.32oN, 3m), Hamburg (53.57oN, 8m), Boulder, Colorado (40.05oN, 1655m) and Kwajalein (8.71oN, 3m).
The difference in back radiation on the average January 1 between the Tropics, Kwajalein, and the Arctic, Barrow, is (411–185) = 226Wm–2 and on the average July 1 it is (421‒308) = 113Wm–2. The summer is warmer than the winter because the average back radiation in summer is 360.5Wm–2 versus 283.5 Wm–2 in winter, Lines 5 and 6 of Table 3.
Description of line items |
Barrow |
Hamburg |
Boulder |
Kwajalein |
|
1 |
Jan. 1 CO2 in dry air, ppmv |
445.1 |
403.1 |
268.8 |
368.8 |
2 |
Jan. 1 after water vapour dilution, ppmv |
444.8 |
400.5 |
264.0 |
358.1 |
3 |
July 1 CO2 in dry air, ppmv |
400.3 |
377.1 |
248.2 |
368.8 |
4 |
July 1 after water vapour dilution, ppmv |
397.4 |
371.0 |
247.0 |
357.6 |
5 |
Jan. 1 back radiation, Wm–2 |
185 |
298 |
240 |
411 |
6 |
July 1 back radiation, Wm–2 |
308 |
368 |
345 |
421 |
7 |
Difference in back radiation, Wm–2 |
123 |
70 |
105 |
52 |
8 |
ΔRF CO2 = 5.22ln(Line 2/Line 4), Wm–2 |
0.59 |
0.40 |
0.35 |
.01 |
9 |
ΔRF CO2 as percent of Δback radiation |
0.48% |
0.57% |
0.33% |
0.02% |
Table 3 Back radiation from the Arctic to the Tropics compared with the warming effect of CO2.
In comparison, the difference in warming by CO2 on the average January 1 between the Tropics and the Arctic acts opposite to back radiation and is ΔRF = 5.22ln(444.8/358.1) = 1.13Wm–2 and on the average July 1 ΔRF = 5.22ln(397.4/357.6) = 0.55Wm–2. In other words, CO2 reduces the January difference of 226 Wm–2 by 1.13 Wm–2, and the July difference by 0.55 Wm–2.
From the Arctic to the Tropics, the warming effect of CO2 is so small at 0.02% to 0.57% of that of back radiation that it has no significant effect on its magnitude.
Figure 4 is the average monthly back radiation in Wm–2 recorded at Hamburg, Germany.16 Back radiation (BR) is a measured value and is the sum of the warming heat radiated back to the Earth by water vapor (WV), CO2, and methane plus the trace gases as in Equation (4).
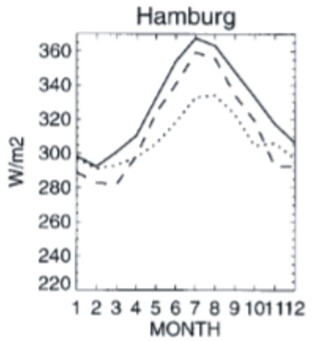
Figure 4 Back radiation from Wild et al.16 The measured values are the solid line. The dotted and dashed lines are computer simulations.
BR = WV ± CO2 ± methane ± trace gases (4)
The signs are ± because they are negative when back radiation is warming the atmosphere as from January to July in Figure 1. From July to January, they are positive as back radiation is cooling the atmosphere.
Rewriting Equation (4) in favor of water vapor gives:
WV = BR ± CO2 ± methane ± trace gases (5)
On the average July 1 at Hamburg, the warming effect of CO2 = 0.6% of that of BR. From Figure SPM.5 of AR5, the warming effect of CO2 is equal to the warming effect of methane plus the trace gases, i.e., 0.6% of BR.
Therefore, WV = BR ± (0.6% x 2) = BR ± 1.2%. Then, the warming by water vapour is approximately 98.8% of the measured value of BR, and the cooling is 101.2% of BR. Thus, the error in Figure 5 is ± 1.2%, which is essentially negligible.
Figure 5 is Figure 1 adapted to show the relationship between water vapour and CO2. Diagrams of the Earth’s energy balance17 indicate the importance of water vapor by showing that on average one half of the sun’s energy reaching the Earth’s surface goes to evaporating water. The back radiation directed towards the Earth’s surface is twice the amount directly from the sun and all of it comes from water vapor. This is serious evidence that water vapor is controlling the Earth’s temperature and climate change.18
Evidence is obtained from applying the laws of physics, namely the gas laws, to the gases in the atmosphere that act as ideal gases to show that from winter to summer CO2 provides a small cooling effect on the atmosphere and from summer to winter it provides a very small warming effect. At the same time, back radiation acts opposite to CO2 and is warming the atmosphere while CO2 is trying to cool it and vice versa. For example, from winter to summer, the warming effect of back radiation at Hamburg, Germany, increases by 70Wm–2, from 298 to 368Wm–2. At the same time, the concentration of CO2 in dry air is falling by 29 ppmv from 403 to 377 ppmv and provides a small cooling effect of 0.40Wm–2, which is 0.6% of the warming by back radiation. From summer to winter back radiation cools the atmosphere by 70Wm–2 and the CO2 provides a small warming effect of 0.40Wm–2. In each situation, the effect of CO2 is so small as to be negligible. Evidence is provided to show the same situation occurs from the Arctic to the Tropics.
Methane and the trace gases also have a similar small cooling effect from winter to summer and vice versa. The sum of their warming effect is the same as that of CO2 (Stocker et al. 2013). Therefore, the warming and cooling effect of all of the greenhouse gases except that of water vapor is ±(2 x 0.06%) = ±1.2% of back radiation. The warming by CO2 plus methane plus the trace gases is small enough to be negligible. All greenhouse gases can warm or cool the atmosphere depending on conditions. An example is shown for water vapor and CO2 in Figure 5.
Typically, the annual inverse relationship between CO2 and water vapor occurs daily. For example, at Montreal, Canada, on the average July 1 the concentration of CO2 from 6 AM to mid–afternoon moves opposite to the temperature rise of 7°C and falls by 9.0 ppmv.
The laws of physics, namely the gas laws, are the key to understanding the very small, essentially negligible, effect that CO2, methane and the trace gases have on atmospheric temperature and climate change. The gas laws give similar results regardless of the concentration of CO2, methane and the trace gases in the atmosphere. From the results of this work, it is clear the government policies to curb fossil fuel consumption and thereby control climate change are ineffectual because CO2 has virtually no effect on atmospheric temperature or climate.
It appears the gas laws as applied in this paper are not included in climate models. If they were included the models could not project continually increasing atmospheric temperature with increasing concentration of CO2. Whether or not the models can be restructured for improved performance is beyond the scope of this study.
None.
Author declares there is no conflict of interest.

©2018 Lightfoot, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.