eISSN: 2577-8307


The community conserved sacred groves which exist over generations, is a testimony for positive anthropogenic intervention in biodiversity conservation. Groves existing across diverse climatic conditions of Central Western Ghats has recorded144 tree species of which 15 were endemic in nature with a high Shanon’s diversity index of 4.15. High tree density (360 trees ha–1) with a basal area of 32.8–49m2 ha–1 is an indication of favorable growing conditions as well as efficient functioning of the groves. This was further substantiated from the typical inverted “J” curve of girth class distribution, indicating the normal regeneration process prevailing in the groves. The carbon sequestered from above ground biomass of standing trees, soil organic carbon and litter was 139.78, 62.3 and 0.38 tC ha–1 respectively. About 196.43 tCha–1 sequestered in the groves is highest so far reported among the forest ecosystems of India. Thus results indicate the importance of community conserved forest ecosystems in sustaining the biodiversity which impart functional diversity and in turn resilience of the ecosystem. Such self sustaining ecosystems under changing climatic conditions are most essential in mitigating climate change.
Keywords: climate change, western ghats, biodiversity conservation, tropical forests
Forests have been fulfilling the human needs from time immemorial. India has a wide range of forest types being in tropical region1 and house rich biological diversity which is evident from the fact that, four out of 34 global biodiversity hotspots are located here.2,3 Sacred groves are one of the forest ecosystems conserved by local communities reinforced by religious sentiments towards the forests in Western Ghats of India. Sacred groves are an example for positive human intervention in conserving forests.4 The ownership of the sacred groves is with the federal government and managed jointly with the local communities. The contributions of local communities towards the managements of sacred groves are mainly in terms of providing protection and tree enrichment. This has contributed immensely in maintaining health and growth of sacred groves and is instrumental in providing many non–tangible benefits such as biodiversity conservation, carbon sequestration, water and soil conservation, aesthetic and recreational services.5 Such ecosystems in the semi–arid tropical countries are of great importance considering the significant land cover changes and socio–economic transformations leading to biodiversity loss and decline in associated ecosystem services. Sacred groves are also prevalent in various manifestations in Europe, North America, Eastern Africa, and China and in few Arabian countries.6
The benefits derived from forests primarily depend upon the ecosystem functioning and the susceptibility to drought, floods, invasions and other climatic aberrations. Ecosystem functioning is shown to increase with increase in the biodiversity in the early nineteenth century itself.7,8 This hypothesis was strengthened in the due course of time.9,10 It is proposed that, higher the number of species in an ecosystem, greater would be the number of inter–specific interactions linking each other and enhance the functional ability of the ecosystem.11 The variations in physiological processes, morphological differences and life histories of plant species12–14 allow mixtures of species to utilize the resources comprehensively and help in optimizing plant productivity.8,15 Thus with more number of plant species in a community there will be more alternate pathways for the internal cycling of nutrients and flow of energy that help in sustaining the stability and productivity of the ecosystem.16 Under the current climate change scenario understanding functional ecosystem response has become more relevant to provide robust interpretations and generalizations10 that help in addressing climate mitigation.
Biodiversity loss is one of the major concerns due to global climate change. Global climate is becoming variable as predicted, due to unabated GHG emissions. As various measures adopted to reduce the emission levels have not yielded the desired results,17 carbon sequestration from forest ecosystems is considered to be a potential means of climate mitigation because of its cost effectiveness18 and many ecosystem services derived. Forest ecosystems have been clearly shown to be one of the important sinks of carbon.19–23 Globally, the combination of reforestation and afforestation could reduce atmospheric CO2 concentrations by as much as 30 ppm in this century.24 The biomass accumulation in the Indian forests has contributed in removing 9.31% of the total annual emissions of year 2000.25 Estimates also show that the continued sequestration by forests would be able to offset 4.87% of the emissions up to year 2020, at which emissions will be 95% higher than that of 2000. The ecosystems such as sacred groves are of special relevance because of their high biodiversity that impart a wide range of functional abilities in terms of stress tolerance, carbon assimilation and optimum resource utilization11 and help in maximizing the carbon sequestration.
The global climate change can be mitigated by addressing issues at the regional levels. Therefore collating carbon stocks of different ecosystems at regional levels is not only useful in making precise estimations but is also essential for making policy interventions in sustaining these ecosystems and there services. In this background present study is an attempt to assess the sacred groves for their contribution towards biodiversity conservation and carbon sequestration.
Kodagu district is situated on the Eastern slopes of the South–West mountain range of Southern India known as “Western Ghats” that extend between 11º 56'–12º 52' N and 75º 22'–76º 11' E. It is a hilly district with elevation ranging from 900 to 1,750 m. It is one of the most densely forested districts of India with 75 per cent of land with tree cover.26 It has 29 % of the geographical area covered with coffee plantations (coffee in India is grown under the partial shade of highly diverse tree species) and 46 per cent by natural forest and around 16 per cent of the forested area is found outside the reserve forest.26 Sacred groves are part of the forest outside the reserve forest with a land area to sacred grove ratio of approximately one sacred grove for every 300 ha. There are 1,214 sacred groves covering an area of about 2,550 hectares spread across three talukas of the Kodagu district.4
The annual rainfall of the district ranges from 1500–5000 mm with a dry spell of three to four months. The mean annual temperature is 24°C and the mean temperature of the coldest month is around20°C and it ranges from 25°C to 31°C during hot months.27 The major soil types of the district are mollisols, alfisols, ultisols, inceptisols, entisols and red soil.28 All these climatic and soil variables across the district provide varied conditions suitable for different types of plant speciesand perhaps result in hosting a rich biological diversity.
From the initial reconnaissance survey it was found that some of the very small sacred groves were very difficult to locate and some of them do not exist. Therefore, groves of size one to five acres wasnot included in the study. The groves were located from the village maps (scale 1:7920) developed by Surveyor General of India, maintained by Office of Assistant Director of Land Records. The selected groves were grouped into five size classes and from each size class 25 per cent of the groves were sampled (Table 1). The details of the location of the study site and the distribution of sampling plots are depicted in Figure 1. Stratified random sampling was adopted for collection of data from the groves. Stratified random sampling is a technique which attempts to restrict the possible samples to those which are “less extreme” by ensuring that all parts of the population are represented in the sample in order to increase the efficiency. In stratified sampling the population of a unit is first divided into disjoint groups. These subgroups, called strata, together they compromise the whole population, so that each stratum a sample, of pre–specified size, is drawn independently in different strata. Then the collections of these samples constitute a stratified sample. Carbon stock estimation ofthe sacred groves was collated from three major pools of carbon namely; the above ground biomass (AGB) of standing trees, litter and the soil. For estimating the carbon in the standing trees non–destructive method of biomass estimation was followed. In each of the identified groves two plots of size 20m x 20m were laid, one in the middle of the grove and the other towards the outer periphery of the grove. Height and diameter at breast height (DBH) of all the tree species with more than 30 cm girth were measured and identified to species level following the taxonomic classifications.29,30 DBH was measured at 1.37m from the ground using the non–stretchablemeasuring tapeand the height was measured using digital clinometer (Haglof, Sweden). In the absence of allometric equations for all the 145 tree species, we used DBH and tree height to estimate the trunk volume31 and volume was multiplied with the wood density of respective tree species32,33 to derive the biomass. To estimate the biomass in the crown, expansion ratio was used34 and 50 percent of the biomass thus derived was considered as carbon content.35
Size classes (acre) |
Madikeri taluka |
Virajpet taluka |
Somwarpet taluka |
||||
|---|---|---|---|---|---|---|---|
No. of groves present |
No. of groves sampled |
No. of groves present |
No. of groves sampled |
No. of groves present |
No. of groves sampled |
||
I |
5–10 |
34 |
9 |
50 |
13 |
28 |
7 |
II |
10–15 |
15 |
4 |
27 |
2 |
7 |
2 |
III |
15–20 |
9 |
2 |
16 |
4 |
7 |
2 |
IV |
20–25 |
5 |
2 |
9 |
2 |
1 |
1 |
V |
>25 |
8 |
5 |
18 |
5 |
11 |
3 |
Total |
71 |
22 |
120 |
26 |
54 |
15 |
|
Table 1 Sacred groves distribution in the Kodagu district with three talukas and the stratified sampling intensity from different size classes
For soil carbon estimation samples were drawn using a core sampler from a depth of 30cm from ground surface.35 Soil was sliced on to the plastic sheet and coarse fragments were removed. For every 20 x 20m plot 4 soil samples were collected from fourcorners of the plot, and all the samples were pooled and mixed thoroughly to get a uniform color composition and one composite sample was drawn for analysis from each plot. Samples were drawn from five groves to represent each size class. Samples were analyzed for organic carbon content as per modified Walkley and Black method.36 The organic carbon content of these soils is considered to be the total carbon content as other carbon forms are in negligible in mollisols, alfisols, ultisols, inceptisols, entisols and red soils.35
To assess the standing litter, four plots of 1 X 1m dimension were laid in each of the sampled grove. Out of four plots laid, two were in the middle of the grove and two towards the outer periphery. Total weight of the litter collected from each plot was weighed in the field using portable electronic field balance. A representative sample was brought to the lab and washed to remove the soil particles, dust and other adhering particles and then dried in the oven at 70ºC. Using the dry weight of litter necessary corrections were made to the total weight of the litter collected and considered as the biomass of standing litter.
To assess the structural composition of the sacred groves, trees were stratified into six size classes in the vertical and horizontal axis using height and DBH of the trees. Species diversity was assessed using the Shanon’s diversity index.37
Regeneration of tree species in the groves was enumerated using 2x2m plot within the 20x20m plots which were laid for tree enumeration. The regeneration classes were based on the plant height. Three size classes were made namely; plants with <40cm, 40.1 to 100 and >100cm in height but less than 30 cm GBH. This classification was followed to assess the survival of treespecies at different seedling stages. Data thus obtained on various parameters were subjected to statistical analysis to determine the significance using MSTAT software.
This study is an attempt to assess the sacred groves and how groves can contribute towards sustaining the growth and stability of the system under changing environmental conditions. In order to assess the functional ability of sacred groves at the landscape level, structural attributes, spatial arrangements, size distribution and regeneration abilities of the ecosystem are considered to be good indicators.38–41 These traits reflect the cumulative effects of energy flow, nutrient cycling and environmental perturbations on the ecosystem performance. The basic premise of ecosystem functioning is the interaction between the biological components and their environment2 where environment will influence and also get influenced by the biological components of the ecosystem. Therefore the productivity of an ecosystem depends on the efficiency with which the interactions occur. Higher the efficiency, higher will be the productivity. Efficient interaction is noticed when the plant diversity is higher.8,15 Because larger the number of species in an ecosystem, greater would be the possibilities of interactions that enable the selection of extreme traits that would enhance the collective performance.11
Biological diversity
One of the major components that play an important role in the functioning of an ecosystem is tree diversity.52 From the results it was found that there are 144 tree species recorded in the sacred groves (online resource Table 1)of which 14 were found to be endemic to sacred groves (online resource Table 2). The tree species richness of the sacred groves is greater than (91) species reported earlier for this region.38 Such large tree diversity is primarily due to diverse climatic conditions prevailing over the district located in the hilly terrain of the central Western Ghats.42,43 It receives a mean annual rainfall ranging from1500 mm in the lower planes to 5000 mm in the higher altitudes.44 The altitude varied 900–1757 m. The rainfall, altitude and its associated changes in soil and physiographic factors bring in lot of climatic variations and resulted in formation of dry deciduous, moist deciduous, semi evergreen and evergreen forests types in the district. Since the sacred groves are spread across the district in these diverse forest types (Figure 1), it is likely that diverse species of different climatic requirements would thrive in these sacred groves. Further, the religious sentiments towards the sacred groves have secured the groves from anthropogenic disturbance also help substantially in sustaining the diversity over generations. The Shanon’s diversity index has remained high and non significant across the size classes of the groves (Table 2), suggests that the tree diversity remained same, irrespective of the size of the sacred groves. The co–existence of species is substantially explained through neutral theory45,46 and niche theories.47,48 Higher diversity is hypothesized to enhance the ecosystem productivity 62. Because larger the number of species, greater would be the interactions of physiological, morphological traits that will help in enhancing the efficiency of ecosystem functioning in subjecting the resources to more efficient use.11 More alternate pathways for flow of energy and internal cycling of nutrients available among the diverse tree species are expressed11 and thus enhancing the resilience of the system to climatic aberrations.
Growth components & carbon pools |
Class-I |
Class-II |
Class-III |
Class-IV |
Class-V |
Mean |
CD@5% |
Shanon’s diversity index |
3.27 |
3.27 |
3.05 |
3.87 |
3.32 |
3.35 |
NS |
Tree density (tree/ha) |
305 |
299 |
431 |
360 |
405 |
360 |
57.43 |
Basal area (m2ha-1) |
32.80 |
33.30 |
42.90 |
49.00 |
40.60 |
39.70 |
08.24 |
AGB (t ha-1) |
254.00 |
280.00 |
350.00 |
334.00 |
332.00 |
310.00 |
76.74 |
C- content of AGB (t ha-1) |
114.00 |
126.00 |
158.50 |
150.70 |
149.70 |
139.58 |
38.36 |
C-content of Litter (tCha-1) |
0.29 |
0.33 |
0.28 |
0.35 |
0.65 |
0.38 |
0.038 |
Soil Organic Carbon content (tCha-1) |
113 |
124 |
123 |
139 |
126 |
125 |
NS |
Total carbon (tCha-1) |
170.29 |
189.05 |
219.26 |
190.49 |
213.07 |
196.43 |
NS |
Table 2 Tree distribution, Growth and Carbon stocks in major carbon pools in the sacred groves
Note: values given in the parenthesis are the carbon content expressed in percent.
Structural composition of sacred groves
Most important ecosystem function that provides primary energy required for life is photosynthesis. This function of the ecosystem depends on the efficiency with which the solar energy is utilized. In this context the composition and structure of the ecosystem is a key factor according to niche theory.48 Accordingly a strong growth–strategic differentiation at varying light levels has been reported across the species.49 Thus in order to understand the structural composition and its influence on functional ability of sacred groves the spatial arrangement of trees was analyzed. Results showed that the number of trees in the lowest (1–5m) and highest height classes (>25m) remained least among all the size classes of the groves and highest number of trees were found in the height classes ranging between 5.1 to 20m (Figure 2). The mean height distribution of trees in all the size classes of the sacred groves also had a similar trend in which one and five percent of the total population was found in lowest and highest height classes respectively, followed by 28, 30, 21 and 13 percent of the population in the other height classes (Figure 2). This suggests that the canopy species as well as the shade tolerant species are less in number compared to those that need moderate light. Such an arrangement of species depending on their light saturations would optimize the light use efficiency of the ecosystem39,47 resulting in good growth. This was evident from many of the growth attributes such as biomass accumulation, girth increment, distribution, tree density and regeneration as described below.
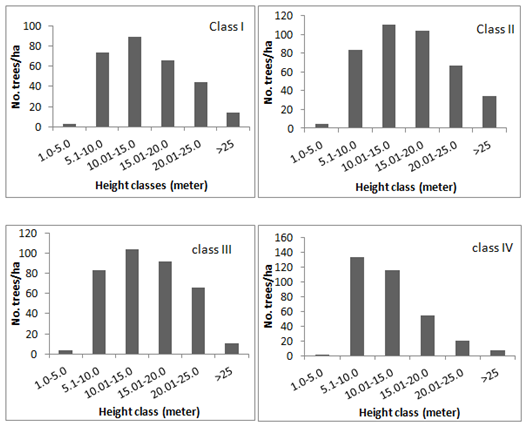
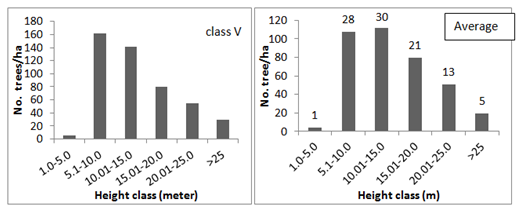
Figure 2 Height class distribution of trees in different size class of the sacred groves (values indicated above the bars in the graph of average tree height is the percentage of tree population).
The highest numbers of trees were seen among the lowest diameter class and the number decreased with increased DBH (Figure 3). The mean distribution pattern of number of trees of different diameter classes was no different wherein, about 48 percent of the total population was found in the lowest DBH class (0.31–0.60 m) and decreased in the subsequent diameter classes continuously to as low as seven percent in the highest diameter class (Figure 3). This has resulted in classical“inverted J” pattern of distribution of diameter classes of trees which is conventionally considered to be a reflection of normal growing conditions of an uneven aged forest ecosystem.3 The subtle climatic variations seen in the vertical and horizontal profiles of the ecosystem provide variable climatic conditions congenial for diverse species, enabling many species to co–exist.47,48,50


Figure 3 Girth class (DBH) distribution of trees among the size class of the sacred groves (the values indicated above the bars in the graph depicting the average DBH is the percentage).
When the conditions are favorable for growth it should also be reflected in the tree population. Tree density in the groves varied from 299 to 431 tree/ha across the size class of the groves, with an average 360 tree ha–1 (Figure 2). Tree density recorded in deciduous forest type of the district is as low as 67 tree ha–1 and highest recorded is 270 treeha–1 in semi evergreen forest type.18 In a managed ecosystem of the district viz; coffee plantation (coffee is grown under the partial shade of trees in India) shade tree density is reported to vary from 370 tree ha–1 in evergreen vegetation type to 361 trees ha–1 in moist deciduous vegetation type of the district41. Thus it is evident that the tree density of sacred groves is on par with a managed ecosystem and much higher than a natural forest ecosystem, reiterating the fact that the growing conditions prevalent in the sacred groves are congenial for growth. This was also evident from the natural regeneration of the sacred groves (Figure 4). The average number of regeneration in three size classes of regeneration was 9663, 8515 and 5369 plants/hectare in the <40, 40.1 to 100 and >100 cm height classes respectively. The number of individuals decreasing progressively with size of the recruits is a sign of healthy growing environment10 and is also indicative of the fact that conditions in the sacred groves are favorable for natural regeneration. Tree density can also be attributed to tree planting of certain species by the local communities.
Carbon sequestration
The biomass and carbon stocked in thestanding trees ranged from 228–316 t ha–1 and 114–158 tha–1 respectively, with significant differences among the size classes. The average amount of carbon stored is 139.78 tones ha–1 (Table 2). Biomass accumulation in evergreen, semi evergreen and deciduous forests in India is 183.06, 181.73 and 105.2 tones ha–1 respectively.51 In tropical dry evergreen forests of peninsular India it ranged from 36.69 to 170.02 tones ha–1,52 while that of tropical rain forest of Uttara Kannada district of Western Ghats ranged from 92 to 268.49 t ha–1.53 Average biomass accumulation of Indian forests is 135.6 t ha–1 (Pandey 2002).7 Highest biomass of 397.7 t ha–1 is reported from the Amazonian forests.54 These comparisons suggest that the sacred groves have highest carbon stock in the standing trees among the forest ecosystems of India. Such a high productivity seen in the sacred groves can be largely attributed to the high species diversity as well as its distribution in the groves. Because, according to11 higher diversity can increase productivity as the probability of having highly productive species presence will increase with plant diversity (competition effect) and complementary resource use by different species would also be higher with plant diversity.
From the size class distribution and their respective biomass contribution it was found that the number of individuals present in different girth classes decreased with the increase in girth, while the biomass contribution from these respective size classes was exactly the opposite (Figure 5). This is a general trend seen in most mixed forest ecosystems53,55 where the largest contribution towards biomass comes from (larger trees) old growth whose number invariably remainsless.

Figure 5 Size class distribution of tree population in the sacred groves and the amount of carbon stored in the respective girth classes.
The quantum of carbon present in the standing litter is in the range of 0.28 to 0.65 tones ha–1 (Table 2), which is relatively less compared to earlier reports for Western Ghats56,57 and also considering the higher tree density of the groves. These are the average values across the groves which are distributed across the varying climatic conditions with a composition of both evergreen and deciduous types. Therefore litter fall and its mineralization can be both seasonal (in deciduous species) as well as continuous (in evergreen species) among the groves.58 These factors must have been the reasons. Since this was not a major focus of this study, more detailed investigations are necessary.
Soil organic carbon (SOC) content of the groves varied from 56 (1.25%) to 69.45(1.55%) tones ha–1 across the groves with an average of 62.45(1.4%) tones ha–1 (Table 2). The average soil carbon content of the Kodagu district is reported to be in the range of 0.5 to 0.75 %.59 The values reported for sacred groves were found to be higher, compared to dry deciduous forest (42 tones ha–1) and semi–evergreen forests (63.12 tones ha–1) of the Western Ghats.51 Another major land use system of the region is coffee plantations and SOC values reported are in the range of 56 t/ha (Coffea arabica)to 33.6 t/ha (Coffearobusta)60,28 which is less than that of sacred groves. Higher SOC in the groves can be ascribed to high tree density and diversity and is also an indication of low anthropogenic interference. This is an indication of favorable soil conditions in the groves which is essential for normal growth and development of the trees.
The total carbon sequestered from all the three major pools varied from 170 to 213 tones ha–1 with a mean of 196 tones ha–1. This is considerably higher among all the forest ecosystems of the Western Ghats reported. Largest contribution among the three pools come from AGB followed by soil carbon and litter reiterate the fact that the growing conditions in the groves are favorable and stable.
Tree growth is a function of ability with which solar energy is utilized in assimilating atmospheric carbon to produce carbohydrates, which are building blocks of growth. This process needs to be complemented with essential mineral nutrients. In a closed ecosystem the nutrient turnover is shown to be generally not limiting. If so, it becomes evident in the growth, regeneration and other processes of the ecosystems.3,16 The composition of groves (Figure 2) (Figure 3), growth (Table 2) and regeneration (Figure 4) as a cumulative response of the system, it was evident that the health of the sacred groves was good or not limited by the nutrients and therefore we presume that nutrients circulated reasonably well like a closed forest ecosystem in the sacred groves. The contribution of local communities have significant role in sustaining this healthy environment. Moisture is another major growth limiting factor which was found to be not affecting the growth. Kodagu district receives an annual rainfall ranging from 1500 to 5000 mm and is recognized to be a high rainfall region.27 The rainfall distribution is also fairly well spread as this region receives both South–West (June to September) and North–East monsoons61 with only two to three months of dry spells. This suggests that moisture may not be a constraint in growth of sacred groves (Supplementary Data).
From the above observations it is evident that the groves are functionally diverse due to higher tree diversity that facilitate efficient utilization of resources and in turn sustaining a balanced interaction between the environment and the biological entities of the sacred groves. Higher productivity of the groves among the similar natural ecosystems in the region was because of the resilience of the sacred groves which is perhaps lacking in less diverse systems.61–67
This study was supported by the grants No. ES/71/01/2005 funded by Department of Science and Technology, New Delhi, India.
Authors declare there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.