
Mini Review Volume 1 Issue 1
Thermo physical chemical properties of fluids using the free NIST chemistry webbook database
Osama A Marzouk
Regret for the inconvenience: we are taking measures to prevent fraudulent form submissions by extractors and page crawlers. Please type the correct Captcha word to see email ID.

College of Engineering, University of Buraimi, Oman
Correspondence: Osama A Marzouk, University of Buraimi, College of Engineering ,P.O. Box 890, P.C. 512, Al Buraimi, Sultanate of Oman
Received: May 20, 2017 | Published: August 28, 2017
Citation: Marzouk OA. Thermo physical chemical properties of fluids using the free NIST chemistry web book database. Fluid Mech Res Int . 2017;1(1):14-18. DOI: 10.15406/fmrij.2017.01.00003
Download PDF
Abstract
Many engineers, students, teachers, academicians, scientists, and researchers need to know intensive thermal, physical, and chemical properties of a fluid at a certain equilibrium state (as determined by temperature and pressure, for example). Such properties include the density, specific volume, viscosity, specific heat capacity, thermal conductivity, speed of sound, specific enthalpy, specific entropy, and surface tension. This article refers to a powerful database for such information, which is called NIST Chemistry WebBook. It is owned by the National Institute of Standards and Technology (NIST), which is an agency of the United States Department of Commerce. The database is available online free-of-charge and does not require registration for access. Its use is simple, and it offers the user multiple options for the unit to be used for each individual property. An application is presented where different properties (including the density, viscosities, specific heats, thermal conductivity, and speed of sound) for water at its liquid phase are presented at an absolute pressure of 1 bar over a temperature range from 1 °C to 99 °C. Comparisons with two sources support the correctness of the database.
Keywords: Fluids; Properties; Thermal; Physical; Chemical; Water
Abbreviations
IR: Infrared; UV: Ultraviolet (visible); IUPAC: International Union of Pure and Applied Chemistry; InChI: International Chemical Identifier; InChIKey: New Fixed-Length Code that Represents a InChI; CAS: Chemical Abstracts Service; NIST: National Institute of Standards and Technology; SRD: Standard Reference Data
Introduction
Fluids (gases and liquids) are encountered heavily in different specializations of engineering and science. Engineers and researchers may frequently need an easy access to a reliable and rich database of fluid properties at a wide range of conditions during their work. Example uses are the design or modification of plumbing systems, ventilation or air-conditioning systems, structures subject to wind loads, plasma flows, heat rejection methods, heat exchanges, and various chemical processes.
In teaching a course, the data in an appendix of a textbook might be sufficient to meet the need of the instructor and student. However, a much deeper and boarder database is needed to satisfy the scope of practical applications, especially with a multidisciplinary nature or a complex configuration. This work explores one such extensive database, showing how powerful it is. Being freely available online and well-documented increases its value. A specific example for using the database is provided for liquid-water at an ambient pressure. A limited validation is presented for that example as well.
Interested readers are encourages to explore the multitude of facets of the database, which go beyond traditional macroscopic properties, such as density, heat capacity, viscosity, thermal conductivity, specific enthalpy, phase-change temperatures, and enthalpy of combustion. The database provides the users molecular-level or atomic-level data, such as ionization energy, electron affinity, proton affinity, cluster ion binding energies, infrared (IR) spectra, ultraviolet (UV) and visible (Vis) spectrum, constants of diatomic molecules, and gas chromatography.
NIST Chemistry WebBook
The National Institute of Standards and Technology (NIST) developed a program called Standard Reference Data (SRD),1 which covers chemistry, engineering, fluids and condensed phases, material sciences, mathematics, computer sciences, and physics. One component of this program is called NIST Chemistry WebBook,2 which is a rich database that offers several thermo-physical-chemical properties of fluids and other species.
The information in the database spans (as of July 29, 2017) the following categories:
- Thermophysical property data for 74 fluids
- Density
- Specific volume
- Heat capacity at constant pressure
- Heat capacity at constant volume
- Specific enthalpy
- Specific internal energy
- Specific entropy
- Dynamic viscosity
- Thermal conductivity
- Joule-Thomson coefficient
- Surface tension (saturation curve only)
- Speed of sound
- Thermochemical data for over 7,000 organic and small inorganic compounds
- Enthalpy of formation
- Enthalpy of combustion
- Heat capacity
- Entropy
- Phase transition enthalpies and temperatures
- Vapor pressure
- Reaction Thermochemistry data for over 8,000 reactions
- Enthalpy of reaction
- Free energy of reaction
- Ion Energetics data for over 16,000 compounds
- Ionization energy
- Appearance energy
- Electron affinity
- Proton affinity
- Gas basicity
- Cluster ion binding energies
- IR spectra for over 16,000 compounds
- UV/Vis spectrum
- Constants of diatomic molecules (spectroscopic data) for over 600 compounds
- Gas chromatography data for over 27,000 compounds.
The following fluids are among the ones in the NIST Chemistry WebBook database shown in Table 1:
1 |
Water |
31 |
Decane |
2 |
Nitrogen |
32 |
Dodecane |
3 |
Hydrogen |
33 |
Helium |
4 |
Parahydrogen |
34 |
Neon |
5 |
Deuterium |
35 |
Argon |
6 |
Oxygen |
36 |
Krypton |
7 |
Fluorine |
37 |
Xenon |
8 |
Carbon monoxide |
38 |
Ammonia |
9 |
Carbon dioxide |
39 |
Nitrogen trifluoride |
10 |
Dinitrogen monoxide |
40 |
Trichlorofluoromethane (R11) |
11 |
Deuterium oxide |
41 |
Dichlorodifluoromethane (R12) |
12 |
Methanol |
42 |
Chlorotrifluoromethane (R13) |
13 |
Methane |
43 |
Tetrafluoromethane (R14) |
14 |
Ethane |
44 |
Dichlorofluoromethane (R21) |
15 |
Ethene |
45 |
Methane, chlorodifluoro- (R22) |
16 |
Propane |
46 |
Trifluoromethane (R23) |
17 |
Propene |
47 |
Methane, difluoro- (R32) |
18 |
Propyne |
48 |
Fluoromethane (R41) |
19 |
Cyclopropane |
49 |
1,1,2-Trichloro-1,2,2-trifluoroethane (R113) |
20 |
Butane |
50 |
1,2-Dichloro-1,1,2,2-tetrafluoroethane (R114) |
21 |
Isobutane |
51 |
Chloropentafluoroethane (R115) |
22 |
Pentane |
52 |
Hexafluoroethane (R116) |
23 |
2-Methylbutane |
53 |
Ethane, 2,2-dichloro-1,1,1-trifluoro- (R123) |
24 |
2,2-Dimethylpropane |
54 |
Ethane, 1-chloro-1,2,2,2-tetrafluoro- (R124) |
25 |
Hexane |
55 |
Ethane, pentafluoro- (R125) |
26 |
2-Methylpentane |
56 |
Ethane, 1,1,1,2-tetrafluoro- (R134a) |
27 |
Cyclohexane |
57 |
1,1-Dichloro-1-fluoroethane (R141b) |
28 |
Heptane |
58 |
1-Chloro-1,1-difluoroethane (R142b) |
29 |
Octane |
|
|
30 |
Nonane |
|
|
Table 1 List of Fluids in NIST Chemistry WebBook database
For fluids, some temperature-dependent properties can be displayed in a tabular form or as an interactive graph (which can be saved as a PNG image file shown in Figure 1-4. The database provides proper references that support the data presented for each fluid.
Figure 1 A snapshot of the online database (NIST Chemistry WebBook) when selecting a species by its chemical formula.
Figure 2 A snapshot of the online database (NIST Chemistry WebBook) showing the search results for the chemical formula: H2O.
Figure 3 A snapshot of the online database (NIST Chemistry WebBook) after selecting water.

Figure 4 A snapshot of the online database (NIST Chemistry WebBook) when attempting to display the Fluid Properties of water.
One can specify the fluid (or the chemical species in general) by any of five methods as explained in Table 2, with the values of water being given as an example.
Specification Method of a Fluid |
Example for Water |
Name |
Water |
Chemical formula |
H2O |
IUPAC3 identifier string InChI4 |
1S/H2O/h1H2 |
IUPAC identifier string InChIKey5 |
XLYOFNOQVPJJNP-UHFFFAOYSA-N |
CAS6 registry number |
7732-18-5 |
Table 2 The five methods to specify a fluid in NIST Chemistry WebBook
Some Water Properties
We consider here liquid-water as an example fluid and present the variation of four thermophysical properties that are commonly used in some engineering disciplines with temperature at a constant pressure of 1 bar (105 Pa), which is approximately equal to one standard atmosphere (101,325 Pa). The temperature range is from 1 °C to 99 °C. The properties are:
- Density (in kg/m3)
- Dynamic/Absolute viscosity (in centipoises, cP)
- Specific heat at constant pressure (in J/g.°C)
- Thermal conductivity (in W/m.°C)
- Specific volume (in cm3/g)
- Kinematic viscosity (in centistokes, cSt)
- Specific heat at constant volume (in J/g.°C)
- Speed of sound (in m/s)
The following formulas provide conversions factors from other units used for the above properties to the ones used here:7-9
(1)
(2)
(3)
(4)
(5)
(6)
(7)
(8)
(9)
(10)
(11)
(12)
(13)
(14)
(15)
(16)
(17)
(18)
(19)
(20)
(21)
Two of the above eight properties (i.e., the specific volume and the kinematic viscosity) are not independent. Rather, they can be derived from other variables. Depending on the application and context, one may need the value of the derived property rather than the base one or vice versa. For example, the kinematic viscosity is commonly used with lubrication oils, whereas the dynamic viscosity (also called absolute viscosity) is common when casting the momentum-transfer equations for gases. The relation between the specific volume to the density, and between the kinematic viscosity to the dynamic viscosity are given in the two following formulas:
(22)
(23)
The variations are presented graphically in Figures 5-12.
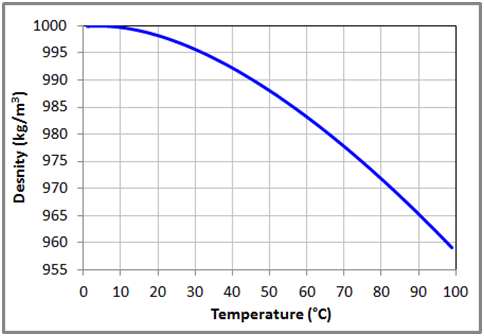
Figure 5 Variation of the density with temperature for liquid water at 1 bar based on the data obtained from NIST Chemistry WebBook.
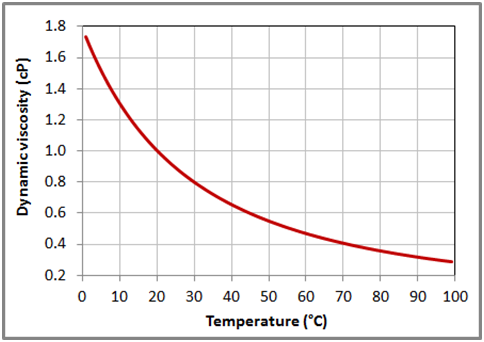
Figure 6 Variation of the dynamic viscosity with temperature for liquid water at 1 bar based on the data obtained from NIST Chemistry WebBook.
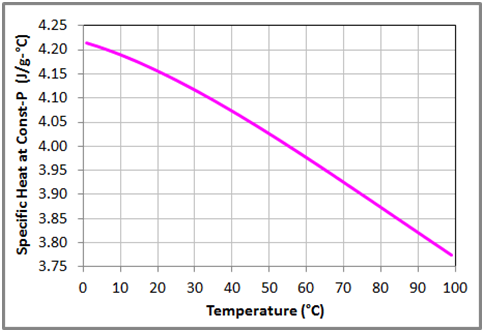
Figure 7 Variation of the specific heat capacity at constant pressure with temperature for liquid water at 1 bar based on the data obtained from NIST Chemistry WebBook.
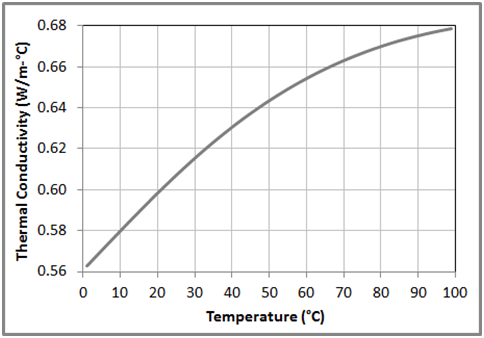
Figure 8 Variation of the thermal conductivity with temperature for liquid water at 1 bar based on the data obtained from NIST Chemistry WebBook.
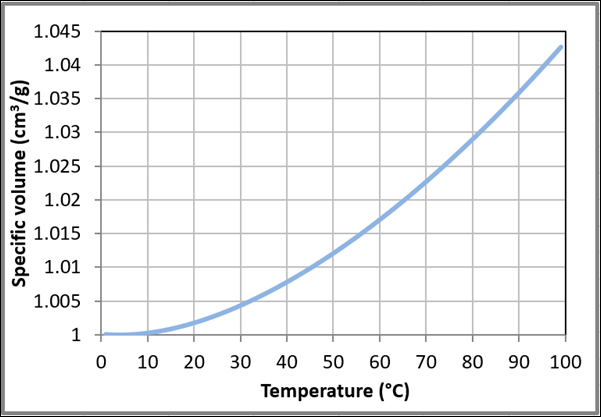
Figure 9 Variation of the specific volume with temperature for liquid water at one bar based on the data obtained from NIST Chemistry WebBook.
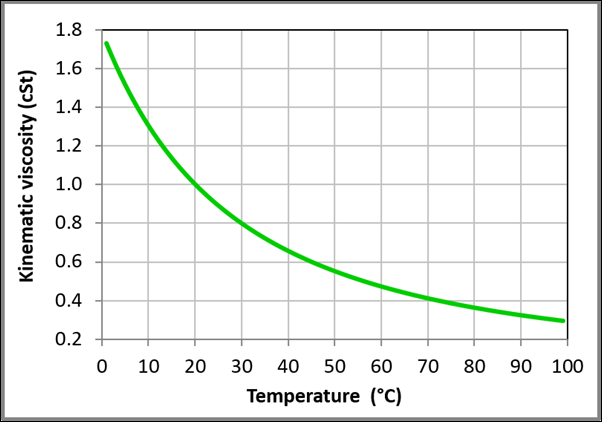
Figure 10 Variation of the kinematic viscosity with temperature for liquid water at one bar based on the data obtained from NIST Chemistry WebBook.
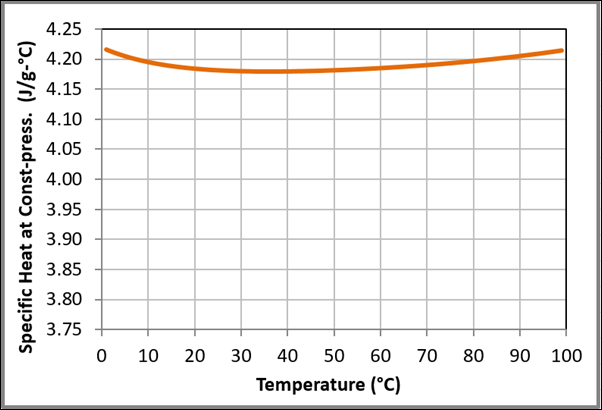
Figure 11 Variation of the specific heat capacity at constant pressure with temperature for liquid water at one bar based on the data obtained from NIST Chemistry WebBook.
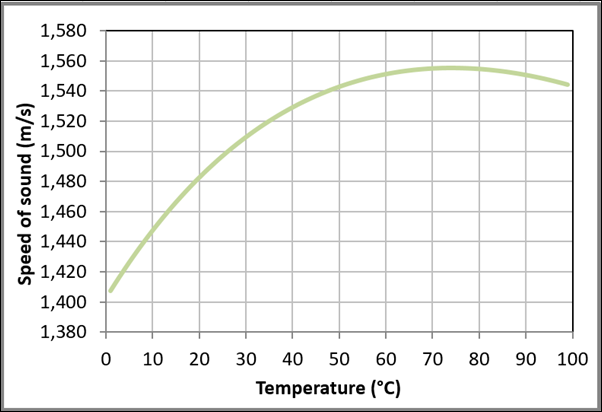
Figure 12 Variation of the speed of sound with temperature for liquid water at one bar based on the data obtained from NIST Chemistry WebBook.
Validation
To judge the accuracy of the values in the NIST Chemistry WebBook, we compare selected values taken for the properties of water as taken from that database with those available in two other sources. The first source is appendix C in a classic reference in the area of water treatment.10 The second source is a software package.11 The comparisons are made at one standard atmospheric pressure (101,325 Pa) for the isobaric (constant-pressure) water data of density in Table 3 and for dynamic viscosity in Table 4. The fluid is water in both tables.
Temperature (°C) |
NIST |
Reference10 |
Reference11 |
10 |
999.7 |
999.7 |
999.8 |
20 |
998.2 |
998.2 |
998.3 |
30 |
995.6 |
995.7 |
995.7 |
40 |
992.2 |
992.2 |
992.3 |
50 |
988 |
988 |
988 |
60 |
983.2 |
983.2 |
983.1 |
70 |
977.8 |
977.8 |
977.6 |
80 |
971.8 |
971.8 |
971.6 |
90 |
965.3 |
965.3 |
965.1 |
Table 3 Comparing some values of the density (in kg/m3) at 101,325 Pa from the NIST Chemistry WebBook and two other sources
Temperature (°C) |
NIST |
Reference10 |
Reference 11 |
10 |
1.306 |
1.307 |
1.306 |
20 |
1.002 |
1.002 |
1.002 |
30 |
0.797 |
0.798 |
0.797 |
40 |
0.653 |
0.653 |
0.653 |
50 |
0.547 |
0.547 |
0.547 |
60 |
0.466 |
0.466 |
0.466 |
70 |
0.404 |
0.404 |
0.404 |
80 |
0.354 |
0.354 |
0.354 |
90 |
0.314 |
0.315 |
0.314 |
Table 4 Comparing some values of the dynamic viscosity (in cP) at 101,325 Pa from the NIST Chemistry WebBook and two other sources
The values taken from the NIST Chemistry WebBook database agree well (and sometimes are identical to) those taken from the other sources. This agreement suggests that the database was prepared carefully and can be used reliably.
Conclusion
This article gave a brief overview of the NIST Chemistry WebBook database, which can be very useful when studying, performing engineering design dealing with, or conducting research on fluids as well as other chemical species. An example was given where the constant-pressure variations of four properties were obtained using that database. A comparison between the values in this online database and other two sources for some properties of water at some conditions showed good agreement. Interested readers are encouraged to explore the capabilities of this free and user-friendly online database.
Acknowledgments
Conflicts of interest
The author declares that there is no conflict of interest.
References
- US Department of Commerce. Standard Reference Data. National Institute of Standards and Technology (NIST), USA.
- US Department of Commerce. NIST Chemistry WebBook. The National Institute of Standards and Technology (NIST), USA.
- US Department of Commerce. Search for Species Data by Chemical Formula. National Institute of Standards and Technology (NIST), USA.
- US Department of Commerce. Search Results for Chemical Formula H2 National Institute of Standards and Technology (NIST), USA.
- US Department of Commerce. Water. National Institute of Standards and Technology (NIST), USA.
- US Department of Commerce. Thermophysical Properties of Water. National Institute of Standards and Technology (NIST), USA.
- MM Khonsari, ER Booser. Applied Tribology: Bearing Design and Lubrication. In: Neale MJ, Priest M, Stachowiak G, editors. 2nd ed. USA: John Wiley & Sons; 2008. 578 p.
- KK Kuo, R Acharya. Applications of Turbulent and Multiphase Combustion. USA: John Wiley & Sons; 2012. 600 p.
- LR Jenkinson, P Simpkin, D Rhodes. Civil Jet Aircraft Design. 1st UK: Butterworth-Heinemann; 1991. 432 p.
- JC Crittenden, RR Trussell, DW Hand, et al. MWH's Water Treatment: Principles and Design. 3rd USA: John Wiley & Sons; 2012. 1920 p.
- VAXA Software. Properties of Water. Chicago. USA; 2017.

©2017 Marzouk. This is an open access article distributed under the terms of the,
which
permits unrestricted use, distribution, and build upon your work non-commercially.











