eISSN: 2577-8242


Technical Paper Volume 3 Issue 2
1Sapat College of Engineering, Management studies and Research, Savitribai Phule Pune University, India
2Baburao Ganpatrao Thakare College of Engineering, Savitribai Phule Pune University, India
Correspondence: YD Kokate, Gokhale Education Society’s R.H. Sapat College of Engineering, Management studies and Research, Nashik 422005. Savitribai Phule Pune University, Nashik, Maharashtra, India, Tel 9822800593
Received: June 15, 2019 | Published: June 24, 2019
Citation: Kokate YD, Sonawane SB. Investigation of particle size effect on thermal conductivity enhancement of distilled water-Al2 O3 nano fluids. Fluid Mech Res Int J. 2019;3(2):55-59. DOI: 10.15406/fmrij.2019.03.00052
Nano fluids are dispersions of high thermal conductivity nano particles in a base fluid, and offer potential to improve thermal conductivity of it. Literature data on the effect of particle size on the thermal conductivity enhancement of nano fluids are inconsistent and limited. Hence in the present study, the effect of particle size on thermal conductivity enhancement of distilled water- Al2O3 nano fluid has been investigated experimentally using transient hot wire apparatus. The particle volume concentration of Al2O3 nano particles for mean diameter of 15nm and 60nm are varied between 0.1 and 3%. The enhancement in the thermal conductivity is approximately 22% and 17% for 15 nm and 60nm particle size respectively, at 3% particle volume concentration at 30οC. These experimental results are consistent with the predictions from the Brownian motion based model, show that the enhancement in the thermal conductivity of the distilled water- Al2O3 nano fluid decreases with an increase in the particle size.
Keywords: thermal conductivity, brownian motion, particle size effect
k thermal conductivity (W/mK) applied electric power per unit length of the wire (W/m)
I electric current (Ampere)
R resistance of platinum wire (Ω)
L length of wire (meter)
p electric resistivity of platinum wire (Ωm)
A Across section area of platinum wire (m2)
Dp diameter of platinum wire (meter)
V voltage (volts)
α Temperature coefficient of resistance (/ºC)
Nano fluids are a new generation fluids synthesized by suspending nanometer-sized materials (nano particles, nano fibers, nano tubes, nano wires, nano rods, nano sheet) in the conventional base fluids. Nano fluids possess immense potential to improve the heat transfer rate and energy efficiency in several thermal engineering application areas including vehicular cooling in transportation, power generation, defense, nuclear, space, microelectronics and biomedical devices.
Several researchers have studied the effect of particle size on the thermal conductivity enhancement of nano fluids. Some of the investigations are briefly summarized here. Xie et al.,1 measured enhancement in the thermal conductivity of ethylene glycol-Al2O3 and pump oil-Al2O3 nano fluids using a transient hot wire method. Five different sizes of Al2O3 particles (~12-302nm) are used in the study. Timofeeva et al.,2 have observed that the water–SiC nano fluids with larger particles of the same volume concentration provides higher thermal conductivity enhancement than those with smaller particles due to smaller solid/liquid interfacial area of larger particles. In contrast, several theoretical3–5 and experimental studies6–8 have shown significant increase in the thermal conductivity enhancement with decrease in the particle size, attributed to Brownian motion. It shows inconsistency in the literature data. Hence in the present study, effect of particle size on thermal conductivity enhancement of water-Al2O3 nano fluid is investigated experimentally.
Synthesis of nano fluid is the first key step in experimental studies with nano fluids. To prepare nano fluids by suspending nano particles into base fluids, ultrasonication and stabilization of the particles are required. For the present work, Al2O3 nano particles of two different sizes (15±5 nm and 60±20 nm) are obtained commercially from Alfa Aesar Pvt. Ltd., India.
There are two basic methods to obtain nano fluids: (a) single step method in which nano particles are produced and dispersed simultaneously in the base fluid and (b) two step method in which nano particles are produced first and then dispersed in the base fluid. A single step method is generally used for nano fluids with metallic nano particles while the two step method is usually employed for nano fluids containing oxide nano particles. The main advantage of the single step method is minimization of nano particle agglomeration. For preparation of water-Al2O3 nano fluids, the two step method is used in the present study. The desired particle volume concentrations used in this study are 0.1%, 0.5%, 1%, 2% and 3%. Water-Al2O3 nano fluid with a required volume concentrations (0.1%, 0.5%, 1%, 2% and 3%) are prepared by dispersing a specified amount of Al2O3 nano particles in water by using a ultrasonic vibrator (Oscar Ultrasonic Pvt. Ltd., India) generating ultrasonic pulses at 34±3 kHz (Figure 1). No surfactant is used in the present study. Several trials are carried out to decide the appropriate sonication time to be used to obtain a stable nano fluid solution. Details of synthesis of the water-Al2O3 nano fluid used in the present work are tabulated in Table 1.
|
Nano fluid |
Sonication time in ( hrs) |
Stable nano fluid kept in test tube |
Period of stable suspension after ultrasonication (hrs) |
|
Water+0.1% Al2O3 (15nm) |
5 |
1 |
24 |
|
Water+0.5% Al2O3 (15nm) |
6 |
2 |
24 |
|
Water+1% Al2O3 (15nm) |
6.5 |
3 |
24 |
|
Water+2% Al2O3 (15nm) |
7 |
4 |
12 |
|
Water+3% Al2O3 (15nm) |
8 |
5 |
12 |
|
Water+0.1% Al2O3 (60nm) |
5 |
6 |
24 |
|
Water+0.5% Al2O3 (60nm) |
6 |
7 |
24 |
|
Water+1% Al2O3 (60nm) |
6.5 |
8 |
24 |
|
Water+2% Al2O3 (60nm) |
7.5 |
9 |
12 |
|
Water+3% Al2O3 (60nm) |
8 |
10 |
12 |
Table 1 Details of synthesis
Figure 2 shows water based nano fluid containing Al2O3 (0.1%, 0.5%, 1%, 2% and 3 % volume concentration) of particle size 15 nm, after sonication (specified in Table 1). Test tube number 1, 2, 3, 4 and 5 indicates 0.1%, 0.5%, 1%, 2% and 3% particle volume concentration of water-Al2O3 (15 nm) nano fluid.
Experimental apparatus
A transient hot wire system is designed, developed and manufactured in the laboratory to measure the effective thermal conductivity of water-Al2O3 nano fluid of particle volume concentrations ranging from 0.1 to 3%. It is most widely used technique for measurement of the thermal conductivity of fluids9,10 This technique gives accurate, fast measurement and also eliminates the effect of natural convection. A schematic diagram and photograph of transient hot wire apparatus is shown in Figure 3 and Figure 4 respectively. An apparatus consists of two measuring cells (each of diameter 50mm), an electrical hardware, a data acquisition system, a power source and a computer. Measuring cells are made of glass. Two similar measuring glass cells of different lengths (10cm and 15cm), connected in series. The diameter of the cylindrical region of the measuring cell is 50 mm. One measuring cell acts as a compensating cell. The formation of three dimensional temperature fields of complex character near the top and bottom ends of the wire are compensated by using a compensating wire.9 The photographs of the measuring cell are shown in Figure 5 Platinum wires of diameter 50.8μm are soldered between brass nuts and bolts in their respective cells. The details of electrical hardware are given.10
Working principle
A cylindrical fluid volume is heated using a current carrying platinum wire, along the axis of the cylinder in this technique. The differential temperature rise of the platinum wire is determined based on the change in the electrical resistance of the wire at different instants. This temperature rise in the wire is plotted against the natural logarithm of the time. The plot is expected to be linear with a slope that varies inversely with the thermal conductivity of the liquid.9,10
Data reduction
The procedure followed for determination of thermal conductivity is described using distilled water at a current of 350mA is as follows.
The thermal conductivity (k) of any fluid can be determined using the transient hot wire apparatus as
(1)
where is the applied electric power per unit length of the wire (W/m) and the slope is obtained from the linear portion of a plot of ΔT verses ln(t).9 We have
(2)
(3)
where, is the electrical resistivity of platinum wire = 105×10-9 Ωm,
From Eq. 1
(4)
or
(5)
with
= constant in the experiment
Therefore, (6)
Similar procedure has been carried out for five sets of experiments and the values of the thermal conductivity of distilled water, toluene and water- Al2O3 nano fluid are determined. The results are presented in the next section.
Before the thermal conductivity of water- Al2O3 nano fluid is measured, the thermal conductivity of distilled water and toluene are measured at 30oC to validate the transient hot wire system. Five sets of experiments are carried out at different currents to measure the thermal conductivity of distilled water and toluene. The values of thermal conductivities of distilled water and toluene are presented in Table 2 and Table 3 respectively. The maximum uncertainty in the measurement of the thermal conductivity is 1.23%. The average over the twenty values from the five sets of experiments gives the thermal conductivity of distilled water as 0.6154 W/m-K, with a standard deviation of 0.027. The present measurement shows excellent agreement with the experimental measurement by Ramires et al.,11 who reported a value of 0.6150±0.0006 W/m-K at 30oC. The average over the fifteen values from the five sets of experiments gives the thermal conductivity of toluene as 0.1306 W/m-K, with a standard deviation of 0.002. The present measurement shows excellent agreement with the experimental measurement by Ramires et al.,11 who reported a value of 0.129±0.01 W/m-K at 30oC. After benchmarking of the experimental set-up, the thermal conductivity of water- Al2O3 nano fluid is measured for different volume concentrations (0.1%-3%) and at 30oC.
|
Current |
Set 1 |
Set 2 |
Set 3 |
Set 4 |
Set 5 |
|
250 mA |
0.6438 |
0.6018 |
0.6104 |
0.6349 |
0.6361 |
|
300 mA |
0.6274 |
0.6379 |
0.5918 |
0.5865 |
0.5909 |
|
350 mA |
0.6243 |
0.5791 |
0.5953 |
0.6585 |
0.6048 |
|
400 mA |
0.6298 |
0.6221 |
0.6254 |
0.5505 |
0.6572 |
|
Average |
0.6313 |
0.6102 |
0.6057 |
0.6076 |
0.6222 |
Table 2 Thermal conductivity of distilled water at different currents
|
Current |
Set 1 |
Set 2 |
Set 3 |
Set 4 |
Set 5 |
|
150 mA |
0.1312 |
0.1296 |
0.1281 |
||
|
200 mA |
0.1327 |
0.1295 |
0.1258 |
||
|
250 mA |
0.1282 |
0.133 |
0.1308 |
||
|
Average |
0.1307 |
|
0.1307 |
|
0.1283 |
Table 3 Thermal conductivity of toluene at different currents
Effect of particle concentration (ø)
The thermal conductivity enhancement of water- Al2O3 (15nm) nano fluid as a function of particle volume concentration at 30ºC is presented in Figure 6. It can be observed from Figure 6 that the thermal conductivity of the water- Al2O3 nano fluid increases with increasing particle volume concentration. In particular, the enhancements in the thermal conductivity of water- Al2O3 (15nm) nano fluid are approximately 3.4%, 9.7%, 15.7%, 18.5% and 22.3 %, found at particle volume concentrations of 0.1%, 0.5%, 1%, 2% and 3% respectively. The corresponding enhancements in the thermal conductivity of water- Al2O3 (60 nm) are 1.31, 5.5, 8.6, 13.6 and 17.6 %, observed at particle volume concentrations of 0.1%, 0.5%, 1%, 2% and 3% respectively (Figure 7).
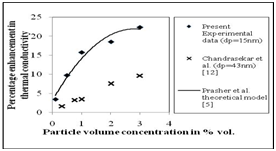
Figure 6 Enhancement in the thermal conductivity of water-Al2O3 (15 nm) nanofluid as a function of particle concentration.
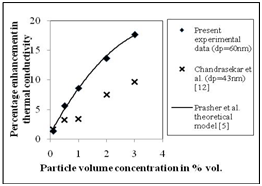
Figure 7 Enhancement in the thermal conductivity of water-Al2O3 (60nm) nanofluid as a function of particle concentration.
Figure 6 and Figure 7 also compare the experimental trends at different particle volume concentration with those predicted by a theoretical Brownian motion based model by Prasher et al.5 and experimental data of Chandraseker et al.12 It can be observed from Figure 6 and Figure 7 that the present experimental data well matches with that predicted by Prasher et al.5 However Chandraseker et al.,12 under predicts the thermal conductivity enhancement of water- Al2O3 nano fluid. The particle size of the Al2O3 is the possible reason for this deviation.
Effect of particle size
The effect of particle size on the thermal conductivity enhancement of water- Al2O3 nano fluid is studied using Al2O3 particle sizes of 15nm and 60nm. The corresponding experimental results are presented in Figure 8. It can be observed from Figure 8 that the enhancement in the thermal conductivity of the nano fluid decreases with an increase in the particle size. These experimental results are consistent with the predictions from Brownian motion based model.5 According to the Brownian motion based model, smaller particles experience higher micro-convection effects which results into higher enhancement in the thermal conductivity. The present experimental data seems to support such models, with a better quantitative match.
The effect of particle size and volume concentration on the thermal conductivity of water- Al2O3 nano fluids have been evaluated experimentally in the present study. The thermal conductivity of the water- Al2O3 nano fluid increases with increasing particle volume concentration and decreases with an increase in the particle size. It indicates that the smaller particles experience higher micro-convection effects which results into higher enhancement in the thermal conductivity. The present experimental data seems to support Brownian motion based model, with a better quantitative match. Hence in order to use the nano fluid for the particular heat transfer application, the selection of the particle size is an important economical aspect for the design of thermal systems.
None.
The author declares there is no conflicts of interest.

©2019 Kokate, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.