
Technical Paper Volume 2 Issue 4
A new approach to modeling and simulation of mass transfer processes in industrial column apparatuses
Christo Boyadjiev
Regret for the inconvenience: we are taking measures to prevent fraudulent form submissions by extractors and page crawlers. Please type the correct Captcha word to see email ID.

Institute of Chemical Engineering, Bulgarian Academy of Sciences, Bulgaria
Correspondence: Christo Boyadjiev, Institute of Chemical Engineering, Sofia 1113, Akad. St. Angelov str., bl.103, Bulgaria, Tel (+359-2)979-32-75, Fax (+359-2)870-75-23
Received: May 15, 2018 | Published: August 2, 2018
Citation: Boyadjiev C. A new approach to modeling and simulation of mass transfer processes in industrial column apparatuses. Fluid Mech Res Int. 2018;2(4):156-162. DOI: 10.15406/fmrij.2018.02.00033
Download PDF
Abstract
In the paper is presented a theoretical analysis of the role of the reaction kinetics in chemical engineering for the solution of the main problems in the chemical industry (biotechnology, heat energy), i.e. the optimal design of new devices and the optimal control of active processes. The thermodynamic and hydrodynamic approximations for the modeling of the industrial process rates are presented and analyzed. The linear and non-linear theories are examined. A new approach to modeling and simulating of mass transfer processes in industrial column apparatuses is proposed. This approach is used for the modeling of chemical, absorption, adsorption and catalytic processes in industrial column apparatuses.
Keywords: reaction kinetics, thermodynamic approximation, hydrodynamic approximation, modeling, simulation, mass transfer, column apparatuses
Introduction
The industrial processes are the result of deviations of the systems from their thermodynamic equilibrium and represent the movement of the systems to their thermodynamic equilibrium. In the chemical industry (thermal engineering and biotechnology too) the deviation from thermodynamic equilibrium is a result of reactions. The reactions are the creation or disappearance of a substance in elementary physical volume (equivalent mathematical point) that is in the phase volume (homogeneous) or on the boundary between two phases (heterogeneous).
In industrial column apparatuses the phase velocities and phase boundaries are unknown. This necessitates the creation of a new approach to mass transfer process modeling, where the velocities are replaced by average velocities and the surface reactions are replaced by volume reactions.
Modeling and simulation
The modeling and simulation are a basic approach in the human knowledge and the science for the quantitative description of the processes and phenomena,1−3 using the combination of intuition and logic.4−5 In the mathematics the intuitions are the axioms (unconditional statements that cannot be proven), while the logic is the theorems (logical consequences of the axioms). The proportion between the logic and the intuition is different in the different sciences. In the mathematics the logic predominates. In the natural sciences (physics, chemistry and biology) the role of the intuition increases, but the "axioms" are not always unconditional. In the humanities the role of the logic decreases.
The modeling and simulation offer quantitative (mathematical) descriptions that have different degrees of detail. The lowest level is the thermodynamic (non-equilibrium thermodynamics) that examines the volume of the phase (gas, liquid, solid). The next level is the hydrodynamic, which examines the elementary phase volumes (mechanics of continua), which are much smaller than the phase volumes, but much larger than the intermolecular volumes, i.e. the molecules are indistinguishable. The highest level is the molecular (the kinetic theory of the ideal gas).
Thermodynamic approximations
The reactions deviates the industrial systems from the thermodynamic equilibrium and the industrial processes for its recovery begin. The determining of the rate of these processes is a major problem in the industry, as it is the basis for their optimal design and control. This gives reason to use the thermodynamic laws of irreversible processes such as mathematical structures in the construction of the process models, described by extensive and intense variables (in the case of merging of two identical systems, the extensive variables double their values, while the intensive variables retain their values).
The kinetics of the irreversible processes uses the mathematical structures, resulting from Onsager's "linearity principle".6 According to him, the mean values of the derivatives at the time of the extensive variables depend linearly on the mean deviations of the conjugated intensive variables from their equilibrium states (values). This principle is valid in the vicinity of the equilibrium, and proportionality coefficients are kinetic (rate) constants.
According to the principle of linearity, the mass derivative at time
[kg-mol.s−1] depends linearly on the deviation from the thermodynamic equilibrium
[kg-mol.m−3] of the concentration in two phase volumes or in one phase and the phase boundary, i.e.
(1)
where
[m3.s−1] is a proportionality coefficient.
Consider a system that contains two identical volumes in one phase
[m3]. The system contains a substance whose masses
[kg-mol] and concentrations
[kg-mol.m−3] are different in two volumes,
. The system is not in thermodynamic equilibrium. Let us assume for certainty
. As a result, the mass of the substance starts to be transferred from volume
to volume
for to achieve the equilibrium. According to the principle of linearity, the mass transfer rate between the two volumes
[kg-moll.s−1] can be represented as:
(2)
where
[kg-mol−1.m3.s−1] is a proportionality coefficient. If we replace masses with concentrations
, the mass transfer rate in one phase
[kg-mol.m−3.s−1] between two points with different concentrations is:
(3)
where
[s−1] is a rate coefficient. This equation is capable of presenting the rate of interphase mass transfer in the case of adsorption or catalytic process, where
is the concentration of the substance in the gas phase, while
is the concentration of the substance in the gaseous portion of the solid (capillaries of the adsorbent or catalyst) phase.
In the cases, where the volumes
are in different phases (for example, 1 is a gas phase and 2 is a liquid phase), the thermodynamic equilibrium law has the form
, i.e. this is the Henry’s law and
is the Henry’s number. If
the mass transfer is from phase 1 to phase 2 and the mass transfer rate between phases is:
(4)
where
[s−1] is the rate coefficient of the interphase mass transfer.
On the surface between two phases, the thermodynamic equilibrium is immediately established, practically, i.e.
, where
, are the equilibrium concentrations on the phase boundary. Thus, the mass transfer rate can be expressed by mass transfer rate in two phases:
, (5)
where
[s−1] are mass transfer rate coefficients.
The Onsager principle of linearity represents the thermodynamic approximation of the mathematical description of the kinetics of irreversible processes, but it does not show the way to reach equilibrium, i.e. the mechanism of the process and as a result the rate coefficient is not known. Obviously, this "thermodynamic level" does not allow a real quantitative description of the kinetics of irreversible processes in industry and the next level of detail of the description, the so-called "hydrodynamic level", should be used.
Hydrodynamic approximation
The processes in the chemical industry and related biotechnologies and heating technologies are realized in one, two and three-phase systems (gas-liquid-solid). They are a result from the reactions, i.e. processes of disappearance or creation of any substance. The reactions are associated with a particular phase and can be homogeneous (occurring in volume of the phase) or heterogeneous (occurring at the interface with another phase). Homogeneous reactions are usually chemical, while heterogeneous reactions may be chemical, catalytic and adsorption. Heterogeneous reaction is the interphase mass transfer too, where on the interphase boundary the substance disappears (created) in one phase and creates (disappears) in the other phase.
The volume reactions lead to different concentrations of the reagents in the phase volumes and as a result two mass transfer processes are realized – convective transfer (caused by the movement of the phases) and diffusion transfer (caused by the concentration gradients in the phases). The mass transfer models are a mass balance in the phases, where components are convective transfer, diffusion transfer and volume reactions (volume mass sources or sings). The surface reactions participate as mass sources or sings in the boundary conditions of the model equations. The models of this complex process are possible to be created on the basis of the mass transfer theory, whose models are created by the models of the hydrodynamics, diffusion and reaction kinetics.
The mass transfer theory combines the chemistry, physics and mathematics and builds its logical structures on three main “axioms”:
- The postulate of Stokes for the linear relationship between the stress and deformation rate, which is the basis of the Newtonian fluid dynamics models;
- The first law of Fick for the linear relationship between the mass flow and the concentration gradient, which is the basis of the linear theory of the mass transfer;
- The first law of Fourier for the linear relationship between the heat flux and the temperature gradient, which is the basis of the linear theories of the heat transfer.
These are the laws of the impulse, mass and energy transfer.
In Boltzmann's kinetic theory of the ideal gas, these axioms are replaced by the “elastic shock” axiom (in a shock between two molecules the direction and the velocity of the movement change, but the sum of their kinetic energies is retained, i.e. there is no loss of kinetic energy) and the rate coefficients are theoretically determined by the average velocity and the average free run of the molecules.
The contemporary mass transfer theory is based on diffusion boundary layer theory.7 This approach substitutes (physically justified) elliptic partial differential equations with parabolic partial differential equations, which facilitates their mathematical solution and offers a mathematical description of physical processes with free (not predetermined) ends.
The diffusion boundary layer theory is developed in the cases of drops and bubbles,8 film flows,9,10 non-linear mass transfer and hydrodynamic stability.11−13
Mass transfer theory
The complex industrial processes are a collection of elementary physical and chemical processes. For example, the chemical absorption in a packed bed column represents a physical absorption of a gas phase component in the liquid phase and a subsequent chemical reaction with a component of the liquid phase. The gas moves in the column like jets and bubbles, while the liquid moves in the form of drops, jets, and flowing films on the surface of the packed bed. As a result, the chemical absorption in a packed bed column is a combination of many elementary physical and chemical processes, as absorption in the systems gas-liquid drops, liquid-gas bubbles, gas-liquid film flow, etc. As an example will be considered the gas absorption in liquid film with free interface.
Let us consider absorption of a slightly soluble gas in a laminar liquid film in a coordinate system (x,y) flowing over a flat vertical interface (y=0).9,10 The hydrodynamic model has the form:
(6)
and the velocity distribution is:
(7)
If these conditions the convection-diffusion model has the form:
(8)
where the thermodynamically equilibrium exists at the film interface
and
denotes the equilibrium concentration. The solid interface (y=0) is impenetrable for the diffusing substance with inlet concentration
(absorption).
Non-linear mass transfer theory
The theory of the diffusion boundary layer2 is the basis of modern linear mass transfer theory, where the convection-diffusion equation in (8) is linear; i. e. the velocity does not depend on the concentration. In a number of cases, the experimental results for the mass transfer rate are higher than the predictions of the linear theory.11,12 This is due to nonlinear effects, where the mass transfer influences the hydrodynamics and the velocity begin to depend on concentrations. These non-linear effects are related to the induction of secondary flows at the interphase boundaries as a result of interphase mass transfer. Such effects are the effect of large concentration gradients,11 the effect of Marangoni and the effect of Stephan flow.12
The large concentration gradients create an intensive diffusion flux that have a hydrodynamic character, and a secondary flow is induces, directed at the normal of the interphase boundary and results in an additional convective mass transfer.
The effect of Marangoni is a result of the gradient of the surface tension on the interphase surface, as a result of the surface gradient of the temperature or surface active agents concentration on the liquid-gas (liquid) interphase, and induces a tangential flow. As a result of the continuity of the flow, there appears to be a much lower flow in the direction of the normal of the interphase boundary and consequently an additional convective flow. Because of this, this effect is relatively weak and occurs in motionless or slow moving fluids.
The Stephan's flow is a result of a phase transition liquid-steam at the interphase surface when the volume of the liquid (steam) increases (decreases) a thousand times. As a result, there is a secondary flow, directed to the normal of the interphase boundary, and an additional convective mass transfer.
In the above three cases, an additional hydrodynamic effect appears very often because the secondary currents disturb the hydrodynamic stability of the flows and self-organizing dissipative structures occur, which further accelerate the mass transfer.9 To these effects can be added the Benar instability in the case of a positive vertical gradient of the density of gases or liquids resulting from concentration or temperature gradients.12
Modeling of industrial mass transfer processes in column apparatuses
The diffusion boundary theory is not applicable for the modeling of chemical, absorption, adsorption and catalytic processes in column apparatuses, where the velocity distributions and interphase boundaries are unknown.
The use of the physical approximations of the mechanics of continua for the interphase mass transfer process modeling in industrial column apparatuses is possible if the mass appearance (disappearance) of the reagents on the interphase surfaces of the elementary physical volumes (as a result of the heterogeneous reactions) are replaced by the mass appearance (disappearance) of the reagents in the same elementary physical volumes (as a result of the equivalent homogenous reactions), i.e. the surface mass sources (sinks), caused by absorption, adsorption or catalytic reactions must be replaced with equivalent volume mass sources (sinks). The solution of this problem is related with the creation of new type of convection-diffusion and average-concentration models.13
The convection-diffusion models permit the qualitative analysis of the processes only, because the velocity distribution in the column is unknown. On this base is possible to be obtained the role of the different physical effect in the process and to reject those processes, whose relative influence is less than 1%, i.e. to be made process mechanism identification.
The average-concentration models are obtained from the convection-diffusion models, where average velocities and concentrations are introduced. The velocity distributions are introduced by the parameters in the model, which must to be determined experimentally.
Convection-diffusion type models
In the general case a multicomponent
and multiphase
for gas, liquid and solid phases) flow in a cylindrical column with radius
and active zone height l [m] will be considered. If
is the fluid flow rate in the column and
are the phase flow rates [m3.s−1], the parts of the column volume occupied by the gas, liquid and solid phase, respectively, i.e. the phase volumes [m3] in 1 m3 of the column volume (hold-up coefficients of the phases), are:
(9)
The input velocities of the phases in the column
[m.s−1],
are possible to be defined as:
(10)
The physical elementary column volumes contain the elementary phase volumes
and will be presented as mathematical points
in a cylindrical coordinate system (
), where
and
are radial and axial coordinates. As a result, the mathematical point
is equivalent to the elementary phase volumes, too.
The concentrations [kg-mol.m−3] of the reagents (components of the phases) are
, i.e. the quantities of the reagents (kg-mol) in 1 m3 of the phase volumes in the column.
In the cases of a stationary motion of fluids in cylindrical column apparatus,
[m.s−1] are the axial and radial velocity components of the phases in the elementary phase volumes.
The volume reactions [kg-mol.m−3.s−1] in the phases (homogeneous chemical reactions and heterogeneous reactions, as a volume mass source or sink in the phase volumes in the column) are
.The reagent concentrations in the elementary phase volumes increase
or decrease (
) and the reaction rates
are determined by these concentrations
[kg-mol.m−3].
The convective transfer in column apparatus is caused by a laminar or turbulent (as a result of large-scale turbulent pulsations) flow. In the elementary phase volume around the point M (r, z) in the column, the mass transfer rate in this volume [kg-mol.m−3.s−1], as a result of the convection is:
(11)
i.e. convective transfer rate in 1 m3 of the phase volume.
The molecular or turbulent (caused by small-scale turbulent pulsations) diffusive transfer rate [kg-mol.m−3.s−1] is:
(12)
i.e., diffusive transfer rate in 1 m3 of the phase volume and Dij [m2.s−1] are the diffusivities of the reagents
in the phases
.
The mathematical model of the processes in the column apparatuses, in the physical approximations of the mechanics of continua, represents the mass balances in the phase volumes (phase parts in the elementary column volume) between the convective transfer, the diffusive transfer and the volume mass sources (sinks). The sum total of these three effects (in the cases of stationary processes) is equal to zero:
(13)
The axial and radial velocity components
and
satisfy the continuity equations
(14)
The model of the mass transfer processes in the column apparatuses includes boundary conditions, which express symmetric concentrations distributions
, impenetrability of the column wall
, constant input concentrations
and mass balances at the column input
:
(15)
Average-concentration type models
The average-concentration model will be presented on the bases of the convection-diffusion model of the one-phase system in the case of
and a pseudo-first-order chemical reaction:
(16)
The average values of the velocity and concentration at the column cross-sectional area in one-phase systems are:
(17)
The functions
can be presented with the help of the average functions (17):
(18)
where
and
represent the radial non-uniformity of the velocity and concentration and satisfy the following conditions:
(19)
The introduction of the average values of the velocity and concentration (18) in the convection-diffusion model (16) leads to the average-concentration model:
(20)
where
(21)
represents effect of the radial non-uniformity
of the velocity.
Generalized variables
The use of the generalized variables
(22)
leads to:
(23)
(24)
In (23, 24) Fo, Pe and Da are the Fourier, Peclet and Damkohler numbers, respectively:13,19
(25)
In industrial columns the order of magnitude of the parameters values is:
(26)
and the models (23, 24) have convective forms:
(27)
(28)
Very often in industrial conditions, an axial modification of the radial non-uniformity of the axial velocity component is realized:
(29)
and the model (27) has the form:
(30)
Model equation solution
The solution of (27)
in the case
is presented on the Figure 1. This solution
permits to be obtained in (22) the average (conditionally called “theoretical”) concentration distribution
in the column (the points on the Figure 2) and function
(the points on the Figure 3).
From Figure 3 is seen, that the function
is possible to be presented as a quadratic approximation:
(31)
where the (conditionally called “theoretical”) values of
are presented in the Table 1. As a result, in the case of axial modification of the radial non-uniformity of the velocity, the model (27) has the form:
(32)
where the parameters
must be obtained, using experimental data.

Figure 1 Concentration distributions
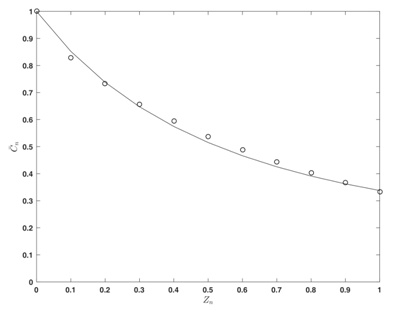
Figure 2 Average concentration distribution: “theoretical” values (as solution of (eq. 27) and (eq. 22))
(points);
as a solution of (eq. 32) for “experimental” values of
(line).
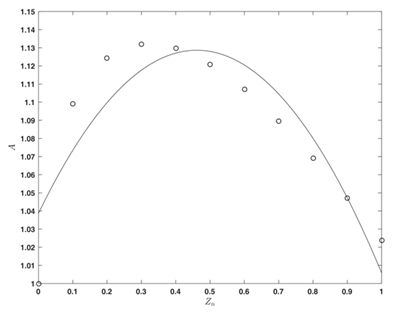
Figure 3 Function
(eq. 22) (points);
as a quadratic approximation (eq. 31) (line).
Parameters |
“Theoretical” values |
“Experimental” values |
a0 |
1.0387 |
0.8582 |
a1 |
0.3901 |
0.4505 |
a2 |
−0.4230 |
−0.4343 |
Table 1 Parameters
Parameter identification
The obtained value of the function
(Figure 2) permit to be obtained the artificial experimental data
for the column end (
):
(33)
where
are obtained by a generator of random numbers. The obtained artificial experimental data (33) are used for the illustration of the parameters
identification in the average concentrations model (32) by the minimization of the least-squares function:
(34)
where the value of
is obtained after the solution of (32) for
,
are the numbers of the artificial experimental data (33). The obtained “experimental” parameter values are presented on the Table 1.
The obtained (“experimental”) parameter values are used for the solution of (32) and the result  (the line) is compared with the average (“theoretical”) concentration values
(points) (as solution of (27) and (22)) on the Figure 2.
(the line) is compared with the average (“theoretical”) concentration values
(points) (as solution of (27) and (22)) on the Figure 2.
Influence of the model parameter
The model (32), with “experimental” parameters values of
in the Table 1, is used for the calculation the average concentrations in the case
and the result (line) is compared (Figure 4) with the average (“theoretical”) concentration values
(as solutions of (27) and (22)) (points) for this case.
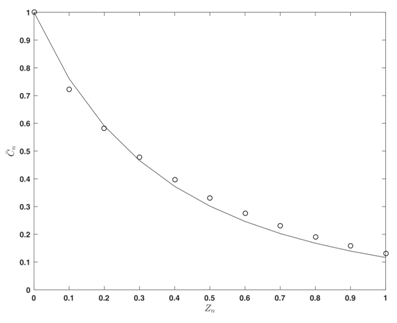
Figure 4 Average concentration distribution
: effect of the chemical reaction rate (
).
Conclusion
The presented numerical analysis of the industrial column chemical reactors shows,14 that average-concentration model, where the radial velocity component is equal to zero (in the cases of a constant velocity radial non-uniformity along the column height), is possible to be used in the cases of an axial modification of the radial non-uniformity of the axial velocity component. The use of experimental data, for the average concentration at the column end, for a concrete process, permits to be obtained the model parameters
, related with the radial non-uniformity of the velocity. These parameter values permit to be used the average concentration model for modeling of different processes (different values of the parameter
, i.e. different values of the column height, average velocity, reagent diffusivity and chemical reaction rate constant).his approach is used for the modeling of chemical, absorption, adsorption and catalytic processes in industrial column apparatuses.14−19
Acknowledgements
Conflict of interest
Author declares there is no conflict of interest in publishing the article.
References
- Chr Boyadjiev. Fundamentals of modeling and simulation in chemical engineering and chemical technology. IHI-BAS, Sofia, Bulgaria; 1993.
- Chr Boyadjiev. Theoretical Chemical Engineering. Modeling and simulation. Berlin, Heidelberg: Springer; 2010.
- Hr Boyadzhiev. Fundamentals of modeling and simulation in chemical industry. International Scientific Journal Mathematical Modeling. 2017;1(1):7−9.
- EL Feinberg. Two cultures; Intuition and logic in art and science. 1992. 251p.
- Chr Boyadjiev. Some Thoughts on Logic and Intuition in Science and Chemical Engineering. Open Access Library Journal. 2014;1(6):1−5.
- J Keizer. Statistical Thermodynamics of Nonequilibrium Processes. New York: Springer; 1987. 506p.
- LD Landau. EM Lifshitz. Fluid Mechanics. 3rd ed. UK: Pergamon press; 1989. 400p.
- VG Levich. Physicochemical Hydrodynamics. New York; Prentice Hall; 1962.
- Chr Boyadjiev, V Beschkov. Mass Transfer in Liquid Film Flows. Moscow: Publ House Bulg; 1984. 128p.
- Chr Boyadjiev, V Beschkov. Mass Transfer in Liquid Film Flows (in Russian). Moscow: Publ House Bulg; 1988. 137p.
- VS Krylov, Chr Boyadjiev. Non-Linear Mass Transfer (Russian). Russia: Institute of Thermophysics; 1996.
- Chr B Boyadjiev, VN Babak. Non-Linear Mass Transfer and Hydrodynamic Stability. New York: ELSEVIER; 2000. 516p.
- Chr Boyadjiev, M Doichinova, B Boyadjiev, et al. Modeling of Column Apparatus Processes. 1st ed. Berlin: Springer; 2016. 313p.
- B Boyadjiev, Chr Boyadjiev. New Models of Industrial Column Chemical Reactors. Bulgarian Chemical Communications. 2017;49(3):706−710.
- B Boyadjiev, Chr Boyadjiev. New Models of Industrial Column Absorbers. 1. Co-current absorption processes. Bulgarian Chemical Communications. 2017;49(3):711−719.
- B Boyadjiev, Chr Boyadjiev. New Models of Industrial Column Absorbers. 2. Counter-current absorption processes. Bulgarian Chemical Communications. 2017;49(3):720−728.
- B Boyadjiev, Chr. Boyadjiev. New Models of Industrial Column Adsorbers. J Eng Thermophysics. 2018;27(1):82−97.
- B Boyadjiev, Chr Boyadjiev. A New Approach for Modeling of Industrial Catalytic Columns. J Eng Thermophysics. (In press). 2018.
- Chr Boyadjiev, M Doichinova, B Boyadjiev, et al. Modeling of Column Apparatus Processes. 2nd ed. Berlin: Springer; 2018. 456p.

©2018 Boyadjiev. This is an open access article distributed under the terms of the,
which
permits unrestricted use, distribution, and build upon your work non-commercially.



![]() (the line) is compared with the average (“theoretical”) concentration values
(points) (as solution of (27) and (22)) on the Figure 2.
(the line) is compared with the average (“theoretical”) concentration values
(points) (as solution of (27) and (22)) on the Figure 2.