eISSN: 2473-0815


Review Article Volume 11 Issue 3
Ministry of Higher Education, Iraq
Correspondence: Saadi J S AlJadir, Ministry of Higher Education, Iraq
Received: October 11, 2023 | Published: November 23, 2023
Citation: Jadir SJSA. The high-density lipoprotein: attribute matters rather than amount in therapeutic arena. Endocrinol Metab Int J. 2023;11(3):61-73 DOI: 10.15406/emij.2023.11.00334
The ability of high-density lipoprotein (HDL-C) to absorb and recycle excess cholesterol from peripheral tissues back to the liver is particularly interesting. This ability may play a role in preventing atherosclerotic cardiovascular diseases, myocardial infarction, transient ischemic attack, and stroke.
Prior epidemiological research has demonstrated that lower HDL-C concentration can be utilized to predict risk and has an inverse relationship with the risk of CVD.
Elevated HDL-C levels are a hallmark of certain hereditary illnesses. However, this does not modulate to a lower risk of CVD. Researchers' focus has been diverted towards the shape and functions of the HDL molecule and its subclasses to correlate the possible causative association between HDL-C and adverse outcomes.
Although a low level of HDL-C is a useful clinical predictor of CAD, raising the HDL-C level does not necessarily lower this risk. The possibility that HDL can either become less effective as an antioxidant or paradoxically enhance the oxidation and inflammation linked to atherosclerotic plaque under certain conditions contributes to the explanation of this dilemma. Thus, the functional properties of HDL, not merely the level, may need to be considered and developed.
On the other hand, the available data indicates that higher HDL-C is not necessarily protective against cardiovascular disease. Conversely, it can be detrimental at extremely high levels. The objective of this review is to elucidate and discuss concisely the current clinical and scientific evidence related to the significance of HDL functionality over the biochemical HDL-C level in mediating the favorable effects on the cardiovascular system.
Keywords: heart, cardiovascular, cholesterol, immunity
ABCA1, ATP-binding cassette transporter 1; ABCG1, ATP binding cassette subfamily G member 1; apoA1, apolipoprotein A1; ApoA2, apolipoprotein A2; CETPi, cholesteryl ester transfer protein (inhibitor); eNOS, endothelial nitric oxide synthase; HDL, high density lipoproteins; HDL-C, HDL cholesterol; TG, triglycerides; LDL, low density lipoprotein; miRNA, micro ribonucleic acid; NO, nitric oxide; NMR, nuclear magnetic resonance; PL, phospholipid; PON-1, paraoxonase-1; RCT, reverse cholesterol transport; CEC, cholesterol export capacity; rHDL, reconstituted HDL; RYGB, roux-en-y gastric bypass; S1P, sphingosine-1-phosphate; S1PR, sphingosine-1-phosphate receptor; SM, sphingomyelin; T2DM, type 2 diabetes mellitus; TNF-α, tumor necrosis factor α; VEGF-A, vascular endothelial growth factor A; VEGFR2, vascular endothelial growth factor receptor 2; VLDL, very low-density lipoproteins; MCP-1, monocyte chemoattractant protein-1; CAD, coronary artery disease; CV, cardiovascular; CIMT, carotid intima-media thickness; AMPK, adenosine monophosphate-activated protein kinase; MI; myocardial infarct; TICE, transintestinal cholesterol excretion
The term “good cholesterol “is often used to refer to the cholesterol content in HDL-C, thus the common trend that we have been accustomed to for decades is to increase HDL-C level and thus achieve cardiovascular protection (Figure 1) (Figure 2).
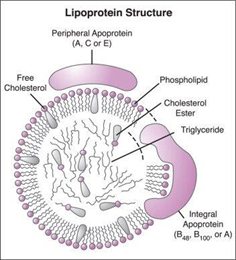
Figure 1 Permission of: Engelking LR. (2014). Textbook of Veterinary Physiological Chemistry. Academic Press.
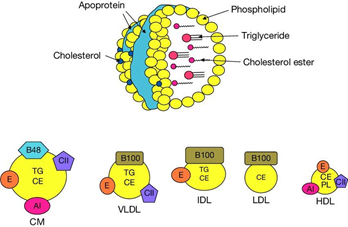
Figure 2 Permission of: Pathophysiology of Human Diseases (pp.1770-1782) Publisher: Elsevier Editors: Linda M McManus, Richard N. Mitchell.
Atherosclerotic cardiovascular disease is known to be the leading cause of death around the world and is accountable for roughly 30% of the annual global mortality rate. In the US, the disease is prevalent with over a third of the population having CVD. Several prospective epidemiological studies have shown a clear inverse relationship between serum HDL-C concentrations and risk for CAD even at target LDL-C (mainly levels below 70 mg/dL). Although lowering LDL-C is much easier than raising HDL-C, it has been shown that for each increase of 1 mg/dL in HDL-C, the CHD risk is decreased by 3% in women and 2% in men.1,2
The particular interest concerning HDL-C is associated with its ability to uptake and re-cycle excess cholesterol from peripheral tissues back to the liver (RCT). Thus, contributes to its role in the prevention of atherosclerosis, myocardial infarction, transient ischemic attack, and stroke. HDL-C levels have been linked to a lower risk of cardiovascular disease and are useful for risk prediction in beforehand epidemiological studies. The first such findings were reported in the Framingham Heart Study during the 80s. Consequently, it was determined that HDL-C is an effective cholesterol carrier that may exhibit protection against CAD. Interventions to increase HDL-C levels, however, have not been shown to provide greater protection against CVD. Furthermore, certain large-scale research findings suggest that HDL-C levels may not necessarily be associated with a lower risk of atherosclerosis in specific clinical situations. It has been discovered that, strangely, elevated CVS risk may also be linked to specific genetic disorders; Primary familial hyperalphalipoproteinemia, endothelial lipase insufficiency, and cholesterol ester transfer protein deficiency, despite having a high sera level of HDL-C.3–7
The ILLUMINATE Phase 3 trial, which used the drug torcetrapib, increased HDL-C levels remarkably through CETPi, which normally catalyzes the transfer of triglycerides from LDL to HDL and cholesterol from HDL to low LDL-C. This study is of significant interest in this aspect. Nevertheless, patients on torcetrapib had an increased risk of adverse cardiovascular events and death more than the atorvastatin-treated cohort (control limb), which led to termination of the trial prematurely.8,9
On the other hand, relatively unsatisfactory results were shown in other clinical trials addressing other CETPi, where raising HDL-C produced neither a significant nor no appreciable effect towards CV endpoints, myocardial infarction, or mortality. In the REVEAL study, Anacetrapib was the only CETPi that showed a modest reduction in major CV events throughout a 4-year follow-up. However, the benefits may have been due to lower LDL-C levels rather than higher HDL (Table 1).
|
|
Anacetrapib |
Evacetrapib |
|
Name (ID) |
REVEAL (NCT01252953) |
ACCELERATE (NCT01687998) |
|
Company |
Merck (Oxford Trial Sponsor |
Eli Lilly |
|
Dose |
100 mg/d |
130 mg/d |
|
Sample size |
30,000 |
12,095 |
|
Inclusion |
1) Age > 50 years |
6) Age > 18 years |
|
2) History of MI |
7) History of ACS (30-365 d) |
|
|
3) Stroke or cerebrovascular revascularization |
8) Cerebrovascular |
|
|
4) PAD repair/revascularization |
9) PAD |
|
|
5) DM with symptomatic CAD |
10) DM with documented CAD |
|
|
Primary end point |
Coronary death, MI, or coronary revascularization |
CV death, MI, stroke, coronary revascularization, or hospitalization for UA |
|
Study duration |
1) Median ~4 years |
3) Median ~2years |
|
2) > 1900 Primary end points |
4) > 1136 Primary endpoints |
Table 1 ClinicalTrials.gov. NCT01252953 ClinicalTrials.gov. NCT01687998. (Accelerate & reveal studies, Comparison of 2 CETPIs)
Beyond CETPi trials results, the fact that HDL is not per se the fundamental cause associated with cardiovascular benefit was supported by Mendelian randomization studies, demonstrating the genetic polymorphism associated with increased HDL has no impact on the risk of myocardial infarction. The results from meta-analyses could not show improvement in cardiovascular outcomes after raising the HDL, on the contrary, was proven to have negative cardiovascular outcomes. The main biological function of HDL is reverse cholesterol transport, whereby HDL accepts and carries excess cholesterol from macrophages in peripheral tissues to recycle it to the liver for disposal through bile excretion to the bowel. All these results open the doors for new avenues to study the quality of whole HDL particle (or particles) rather than merely cholesterol content.10,11
HDLs are a family of particles that can manifest different metabolisms and functions based on their specific proteomic, lipidomic, and physio-chemical properties. Moreover, HDL-C carries various proteins, enzymes, miRNAs, bile acids, and lipids, which all have a potential functional role. HDLs are dynamic particles, being either protective or harmful agents in health or disease respectively. The current research aims to gain a new understanding of HDLs to develop novel therapies and treatments for cardiovascular diseases.
HDL functionality and its relevance to atherosclerotic CVD
Although cholesterol efflux from macrophages accounts only for a very small fraction of the total efflux of cholesterol from peripheral tissues via the RCT pathway, it appears to be the most relevant component regarding cardiovascular protection and therefore it may provide a good surrogate index for HDL functionality (Figure 3).
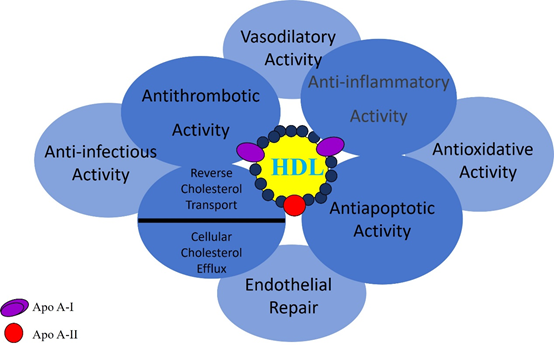
Figure 3 Permission of Chapman MJ, et al. Curr Med Res Opin. 2004;9 Assmann G, et al. Annu Rev Med. 2003;53:321–341.
It was shown in a cross-sectional study that, regardless of HDL-C level, the cholesterol export capacity (CEC) of macrophages has a strong inverse relationship with both CIMT and the relevance of angiographic CAD. Another recent study followed 2,924 adults in the Dallas Heart Study who were free of CVD for more than 9 years. At baseline, HDL-C level, HDL-P concentration, and CEC were assessed. In comparison to the lowest quartile of CEC, there was a significant 67% decrease in cardiovascular risk in the highest quartile. Furthermore, it has been shown that individuals with autoimmune illnesses, diabetes mellitus, metabolic syndrome, and chronic kidney disease have jeopardized CEC. On the contrary, Pioglitazone-treated patients with metabolic syndrome and low HDL-C levels had increased CEC, whereas statin-treated patients with hypercholesterolemia did not show the same boost. Moreover, it has been shown that smoking and having male sex are linked to lower CEC (Figure 4).5,11–15
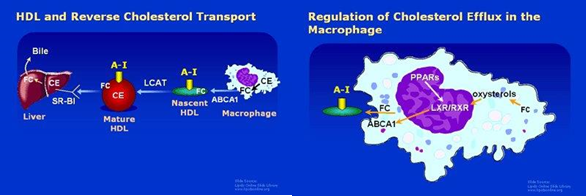
Figure 4 Permission of: J Lipid Res. 2001;16 Atherosclerosis 2002;163(1):1–8.
Thus, quantification of CEC may be beneficial in the assessment of new therapies directed toward HDL metabolism and RCT. These agents that enhance CEC may lead to improvement of HDL functionality and potentially reduction of cardiovascular risk (Figure 5).8
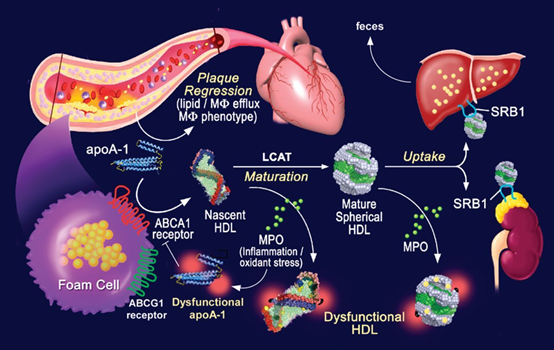
Figure 5 Permission of: Edward A. Fisher. Arteriosclerosis, Thrombosis, and Vascular Biology. High-Density Lipoprotein Function, Dysfunction, and Reverse Cholesterol Transport. 32(12):2813–2820, DOI: (10.1161/ATVBAHA.112.300133). American Heart Association Inc.
Composition of HDL and biological functionality
Basic fact
Cells are incapable of undergoing metabolic pathways to break down cholesterol into smaller molecules to produce energy. For instance, unlike their capability to metabolize other lipids. When a cell accumulates excess cholesterol beyond the optimal quantity for biological functions, it must facilitate the transfer of excess cholesterol to the extracellular media. This biological process is referred to as cholesterol efflux. This is accomplished by a lipoprotein system in which HDL is a main actor, whereby it interacts with cellular cholesterol transporters, partly independent and partly engaging with the apo-B lipoproteins, chylomicrons, VLDL, and LDL (Figure 6).17–19
HDL-C is a lipoprotein consisting of a phospholipid coat that envelops a mainly esterified cholesterol core. The majority of HDL found in plasma is typically spherical, with a size range that allows for significant variation in its cholesterol content. The greater the size of the HDL particle, the higher its cholesterol ester content. Approximately 10% of HDL-C particles display a discoidal shape, characterized by minimal levels of cholesterol ester. HDL possesses proteins on its surface that facilitate a variety of physiological processes within the body. The essential protein found in HDL is apoA1, which provides structural integrity to the particle and promotes the removal of cholesterol from cells to HDL, resulting in the enlargement of the particles. Protein molecules aside from apoA1 may have an impact on the size and structure of HDL, in addition, the phospholipid present in the HDL’s envelope shows significant biological activity. Overall, HDL is an enormous system of particles heterogeneous in size, shape, and amount of cholesterol, and in type of proteins and phospholipids. HDL carries out varied functions; some are related to atherosclerosis while others also have biological actions such as hemostasis, inflammation, anti-oxidation, and inherited immunity.
HDL-C is identified by the presence of apoA1 and a variable size, it circulates in plasma for 2-4 days. Cholesterol that is scavenged by HDL is carried out to the liver for excretion in the bile, and to steroidogenic organs for manufacturing steroid hormones (Vit.D, Gonadal hormones, bile acids, etc.). This cycle of cholesterol from cells to HDL redistributes cholesterol among tissues, in a process called RCT. The potential protection against atherosclerosis, by this achieved process through the reduction of macrophage (foam cells) cholesterol levels in the blood vessels could contribute to the result of the significance of controlling excess cellular cholesterol.
HDL particles subclasses and functionality
HDL-C is a lipoprotein class characterized by its small size, high protein content, and dense structure. It has an average size of 5-17 nm. HDL particles comprise multiple molecules and take variable shapes from spherical to discoid contour, consisting of polar lipids that are solubilized by apolipoproteins. Moreover, HDL comprises many proteins, such as enzymes and acute-phase proteins, many isoforms, and may contain minimal quantities of non-polar lipids (Figure 7).
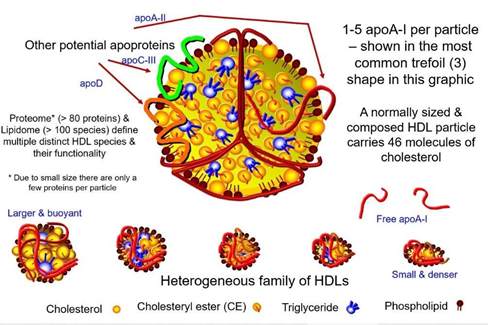
Figure 7 Permission of: Dayspring TD, Toth PP. (2023). Apoproteins and Cell Surface Receptors Regulating Lipoprotein Metabolism in the Setting of Type 2 Diabetes. In: Jenkins AJ, Toth PP, editors. Lipoproteins in Diabetes Mellitus. Contemporary Diabetes. Humana, Cham.
Because of such diverse compositional features, HDL particles are highly heterogeneous in their structural, chemical, and biological properties.
The fundamental structure of HDL is apoA1, which is produced in the liver and bowel. Subsequently, ApoA1 undergoes lipidation through the process of ABCA1-mediated cholesterol efflux, resulting in the formation of nascent discoidal pre-β HDLs. Lipidation, along with the enzymatic conversion of unbound cholesterol to cholesterol esters, facilitates the development of fully formed spherical α-HDL particles (Figure 8).
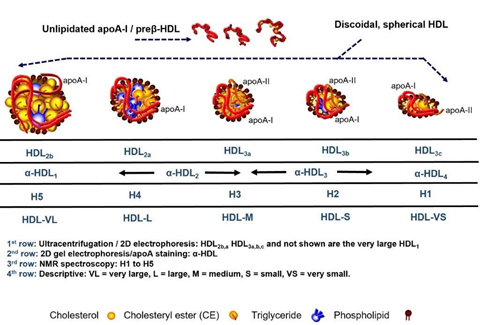
Figure 8 Permission of: Dayspring TD, Toth PP. (2023). Apoproteins and Cell Surface Receptors Regulating Lipoprotein Metabolism in the Setting of Type 2 Diabetes. In: Jenkins AJ, Toth PP, editors. Lipoproteins in Diabetes Mellitus. Contemporary Diabetes. Humana, Cham.
Mature HDL is submitted to invariable dynamic remodeling throughout a 4 to 5-day lifecycle by interaction with a variety of enzymes, such as hepatic and endothelial lipase, producing smaller subspecies (e.g., pre-β HDL) from larger ones (e.g., α-HDL).
This dynamic remodeling of HDL molecules can now be figured out in vivo using newly developed fluorescent probes.20–22
HDLs are complex particles, which can be divided into many subclasses according to their distinctive physicochemical properties.
There is no agreement about the classifications of HDL subclasses or the distinct methods to define them. In conjunction with the diverse techniques of HDL isolation, this will hinder our understanding and ability to define the biology and significance of vascular and metabolic disorders. Experts ranging from basic scientific research to clinical expertise have developed a subclassification for high-density lipoproteins (HDLs) of 5 parts, constituting all confined characteristics: very large HDL, large HDL, medium HDL, small HDL, and very small HDL. The function and metabolism of HDLs can be affected by the related subclass and the ability to differentiate between HDL subclasses and consequently might have clinical significance related directly to the efficacy of statins’ treatment.23–25
Advent's new gold-standard techniques of classification, such as nuclear magnetic resonance (NMR), have the advantage of measuring HDL classes from whole plasma without prior isolation. In addition, tremendous efforts are now stressing on elaborating the complex lipidome, proteome, and structural integrity of HDL particles and subclasses.26,27
We recommend that further clinical studies should establish reference values for the technique adopted; NMR should assess whether integration of HDL subclass measurements and parameters of HDL functionality with patient-specific biomarkers can enhance the stratification of patients for differential diagnosis, disease progression, and responses to therapy.
The lipid composition, size, and structure of HDLs are strongly interrelated.
In patients with type 2 diabetes mellitus, many studies indicate a noticeable change in the reduced size of HDL particles. Moreover, there is an increased content of triglycerides in HDL, potentially leading to increased hydrophobicity. These findings challenge the tendency to express that small HDL particles are constantly protective and instead show otherwise a more compounded relationship between HDL size, composition, function, and the specific disease state. HDLs play an essential role in maintaining the glucose balance inside the body. They achieve this by stimulating the uptake of glucose through the activation of AMPK, a protein that regulates energy metabolism. Moreover, HDLs enhance the secretion of insulin and provide protection against pancreatic ß-cells apoptosis.
Of note, advanced glycation endproducts (AGEs) that result from non-enzymatic modification of proteins that occurs in chronic hyperglycemia leads to glycation of HDL and apo A-1 curbing their functionality by reducing both their CEC and antioxidant properties.28,29
Therefore, type 2 diabetes mellitus has the potential to influence HDL function, and on the other hand, HDL function may influence the development and progression of T2DM.
Furthermore, macrophages myeloperoxidase, which shows an elevation in atherosclerotic CVD, can catalyze harmful changes to the proteins related to HDL, especially apoA1. This will result in the jeopardized ability of RCT and enhance the inflammatory pathway.
Serum amyloid A is a causing factor of HDL dysfunction, inducing a loss of anti-inflammatory and RCT function and curtailing the ability of HDL to interact with the adipocyte plasma membrane. In addition to the functions of HDL-C mentioned, there are additional pivotal functions of HDL in the domain of immunity, prevention of Alzheimer's disease, and enhancement of cancer survival.30
Apo A-1 milano mutation and longevity
The ApoA-I Milano mutation was initially documented in 1980 within a family with roots in Limone Sul Garda, a small locality situated near Milan; North of Italy (Figure 9). In this genetic mutation, the Apo A-I variant exhibits a single amino acid substitution at position 173; cysteine instead of arginine within the primary sequence of Apo A-1. The individuals who possess the ApoA-I Milano Mutation (3% of the entire population of 1000) exhibit a lipid profile that is distinguished by noticeably reduced levels of HDL-C and moderately elevated levels of triglycerides, yet there is no indication of early CAD or subclinical atherosclerotic CAD or CIMT. The scientists in Milano had enabled tracking of the mutation that descended from a single person Giovanni Pomarelli who was born in 1780.13
In the '90s, research conducted at the Cedars-Sinai Medical Center showed that injection of a synthetic ApoA-I Milano into rabbits and mice could reduce vascular plaque load.
Weekly infusions of recombinant ApoA-I Milano, like standard treatment (placebo), resulted in a remarkable reduction in coronary atherosclerosis in patients diagnosed with acute coronary syndrome following only five treatment sessions.
Moreover, in an animal study, it was demonstrated that rApoA-1 Milano showed enhanced anti-inflammatory, antioxidant, and plaque-stabilizing properties in comparison to standard HDL. These cardiovascular protective effects of Apo A-I Milano provided the initial clinical support for the concept that the functionality of HDL plays a more important role in reducing atherosclerosis than the quantitative, circulating level of HDL-C (Figure 10).31
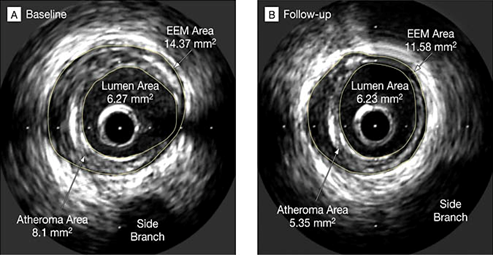
Figure 10 Permission of: Nissen SE, et al. JAMA. 2003.37
The HDL-C efflux capacity; the main functionality of HDL
The varied protein and lipid composition of HDL contributes to its athero-protective function. Within the vascular wall, HDL-C undergoes trans-cytosis across endothelial cells into the subendothelial space. This process allows HDL to remove cholesterol from foam cells, which are macrophages that have accumulated cholesterol. By doing so, HDL helps prevent the formation of plaque. The receptors involved in RCT differ among HDL subtypes. Notably, small pre-β HDL shows a higher affinity for ABCA1-dependent cholesterol export, while α-HDL shows another favorable pathway; ABCG1.
Besides the controlled trials, HDL shows various positive characteristics in the human body. These include their antioxidant ability, stimulating nitric oxide (NO) production, anti-inflammatory effects (specifically, reducing the expression of vascular adhesion molecule -1), and anti-apoptotic actions.32,23
Notably, the most important property of HDL is its ability to induce NO production in endothelial cells, HDL may also stabilize eNOS and delay catabolism.33,34
In patients with atherosclerotic CAD, it has been observed that larger HDL particles show a reduced capacity for antioxidative properties compared to smaller, denser particles. This phenomenon may be attributed to an altered proteome. Larger HDL particles have been found to display a correlation with apolipoprotein A2, a protein that has been shown to reduce the interaction between HDL and PON-1, an enzyme responsible for detoxification that is bound to HDL (Figure11, a & b Zoom in).
Small, dense HDL3 has a more potent anti-inflammatory effect than larger HDL2, explained by their high ability to inhibit TNF-a induced VCAM-1 expression in an endothelial cell culture. In this study, it was determined that proteomic modifications did not play a role in the observed effects. Specifically, the intentional substitution of apolipoprotein 1 with apolipoprotein 2 in HDL3 did not result in any changes to the salutary anti-inflammatory properties. Interestingly, increasing evidence seems to point to a disease-specific HDL size function relationship, while smaller HDLs seem to protect against atherosclerosis. In specific metabolic disorders, like T2DM, larger HDLs seem beneficial, potentially due to improved RCT function or a different molecular composition.
HDLs work as dynamic transporters of circulating microRNA (miRNA) to specific cells, and miRNA may play a significant role in the stabilization of HDL-C. The miRNA function of miR-223 and miR-24 is widely studied in medical settings. It has been found that miR-223 shows an efficient anti-inflammatory property, on the other hand, miR-24 has been accused of enhancing the progression of atherosclerosis. In disease states, the miRNA profile of HDL changes, like the changes observed in HDL function, proteome, and lipidome composition.35
HDL dysfunction in pathophysiological state
It had been shown that HDLs isolated from patients with chronic coronary disease and acute coronary syndrome had markedly reduced efficacy in stimulating NO production in vitro. Moreover, they exhibited pro-oxidative and pro-inflammatory potentials.
Recently, there has been a noticeable correlation between acute coronary syndrome and decreased CEC values as well as reduced levels of HDL S1P and apoA1.
In the situation of chronic kidney disease, the increased link between symmetric dimethylarginine and HDL disturbs the biological function of HDL and immediately contributes to the progression of CVD. This happens by rendering HDL less able to remove cholesterol (RCT) from foam cells and by reducing its anti-inflammatory potential. HDLs from patients with valvular heart disease, including rheumatic heart disease, are pro-inflammatory and cannot couple eNOS, which ultimately leads to impaired endothelial ability to produce NO. Meanwhile, HDLs brought from individuals with T2DM show a curbed ability to produce NO and pro-inflammatory properties (Figure 12).36
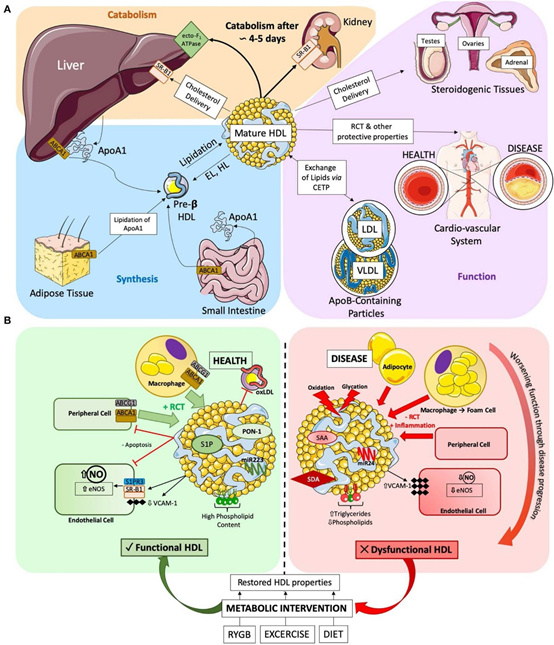
Figure 12 Permission of: Anne Jomad & Elena Asto, High Density Lipoproteins: Metabolism, Function, and Therapeutic Potential, frontiers in cardiovascular medicine, Mar 2020.2
Modifications in the lipidome, such as elevated levels of triglycerides or reduced levels of phospholipids, or coincidental rise in surface rigidity caused by an imbalanced sphingomyelin to cholesterol ratio, decreased RCT capacity of HDLs, as well as their efficacy to bind with beneficial enzymes and proteins.
Recent medical research indicates that the measurement of HDL triglycerides could potentially serve as a valuable biomarker for assessing the quality and function of HDL, increasing the significance of HDL-C. For decades it has been well-accepted that oxidation and glycation of HDLs are the major contributory factors for HDL dysfunction in the human body. Recent data from bariatric surgery has been shown in both human and rodent models. RYGB promotes improvement of HDL function, especially cholesterol efflux capacity, anti-apoptotic, antioxidant, anti-inflammatory activity, and increased capacity to produce NO.
Post-operative follow-up for 12 weeks after RYGB, in comparable groups had shown impaired HDL functions, but gradual improvements in HDL function occurred in the body unrelated directly to weight reduction. Moreover, studies showed that the resumption of HDL function was stable for a long period after surgery.
A matter of extreme interest, biochemical evidence shows that HDLs tend to increase in size following RYGB surgery, therefore adding persistence to the complex relationship between HDL size, composition, and function that have been mentioned earlier.
Regular exercise and dietary adjustments have been found to enhance the healthy functionality of HDLs. Studies have shown a remarkable association between these trials and the extent of weight reduction, in the context of the interpretation of HDLs taken before and after an exercise-targeted weight loss program.
Moreover, various studies have reported an appreciable rise in HDL levels following exercise training. While RYGB appears to exercise its basic influence through supplementary mechanisms.37,38
It is widely known the reduction in body weight has a positive influence on HDLs. This includes an increase in the number of HDL2 particles following dietary modifications, improved ability to remove cholesterol from cells RCT, and changes in the expression of miR223. Moreover, accelerated rise in brown fat metabolism, which has been already compromised in obese individuals, is associated with a positive impact on HDL reshaping, in both human and animal models.
Conditions might render HDL going bad
HDL-C is found in healthy individuals and typically harbors more anti-inflammatory potential. However, in the setting of systemic inflammation (sepsis, infections, etc.), HDL may be submitted to inflammatory changes, as part of the acute phase response’s components
Elective surgery: it had been documented that HDL derived a few days following elective surgery showed a decreased ability to inhibit chemotaxis, as tested by the monocyte chemotaxis assay, in comparison to HDL obtained preoperatively. The impaired HDL restores its efficient anti-inflammatory function following convalescence or post-operative recovery.39
Influenza: virus infection has been reported to exert proinflammatory transformations in HDL.40
Sepsis: has been demonstrated to exert a remarkable influence on the composition of HDL, resulting in a considerable decrease in levels of apo A-I.
Chronic systemic inflammation contributes to chronically impaired HDL function in special patients. This has been observed in patients with coronary heart disease, or coronary risk equivalent (e.g., T2DM, Metabolic syndrome), and hemodialysis patients. In some groups who developed overt coronary disease despite harboring very high HDL-C levels (≥ 84 mg/dL), their HDL showed increased monocyte chemotaxis (as assessed by the monocyte chemotaxis assay) and phospholipid oxidation (as assessed by; Cell-free assay), while HDL from healthy controls had more opposite effects. HDL from a second group with overt coronary disease or coronary risk equivalents who had typical HDL-C levels (mean 57 mg/dL) also showed proinflammatory potential by the same test’s method.16
Recent studies have also documented features of impaired HDL function in individuals having chronic non-vascular inflammation, like rheumatological disorders. Reports have shown that 44% of patients diagnosed with systemic lupus erythematosus and 20% of individuals diagnosed with rheumatoid arthritis demonstrated proinflammatory HDL levels, (as tested by; cell-free assay). In comparison, only 4.1% of the matched-control group exhibited similar proinflammatory HDL levels. These observations may support the concept that women with SLE have a 50 times higher risk of having future myocardial infarction than women without. Moreover, rheumatoid arthritis might double the risk of CAD, despite conventional risk factors having been put under control.41,42
Experimental effects of dietary fats on adhesion molecules (ICAM-1 and VCAM-1)
The report had been taken from a specified study on the lipid content of dietary regimens that remarkably influenced the effect of HDL-C on the expression of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) in vascular endothelium.
Researchers reported that HDL extracted from patients 6 hours postprandially, following meals rich in saturated fat, resulted in a 50% to 80% rise in the levels of these molecules as compared to the basal levels.
On the other hand, HDL that has been taken from individuals who consumed unsaturated fat meals showed a remarkable dip of 50% to 70% in the expression of ICAM-1 and VCAM-1. Interestingly these changes occurred without noticeable alteration in HDL-C levels. We can conclude from this study that saturated fat exerted qualitative alterations in HDL that may potentially enhance monocyte adhesion to the arterial wall.43
Healthy diet and exercise inhibit monocyte chemotaxis:
Scientists studied the effects of a couple of weeks of programs consisting of a dietary adjustment in the form of low fat, high-fiber diet, and an exercise program of 45–60 minutes of treadmill walking. Obese men with metabolic syndrome were involved. Before the program, the participants’ HDL showed proinflammatory properties. The outcome of this program markedly improved the ability of the men’s HDL to inhibit monocyte chemotaxis compared with baseline. This study suggests that healthy lifestyle modifications may affect HDL function, besides the well-known positive effect of exercise on raising HDL-C levels.44
The presumed explanation:
Systemic inflammation may exert specific changes within HDL particles that could hinder their anti-atherosclerotic potential. The best target within HDL in this regard is apo A-I; a pivotal protein involved in RCT, antioxidant, and anti-inflammatory properties. During inflammation, Apo A-I levels fall and can be displaced from HDL particles by acute phase reactants such as serum amyloid A. Apo A-I can also be jeopardized chemically by myeloperoxidase.
Inflammation and its associated oxidative stress can also result in a fall in levels of protective antioxidant enzymes like paraoxonase, besides abnormal accumulation of oxidized phospholipids within HDL. These changes result in an elevation of oxidized LDL particles, which will lead to enhancing multiple aspects of inflammatory pathways within the vascular endothelium (Table 2).
|
Proven to promote the anti-inflammatory effect of HDL |
|
Apolipoprotein (apo) A-I mimetics* |
|
Exercise, low-fat diet |
|
Polyunsaturated fat diet |
|
Statins |
|
May Promote HDL’s anti-inflammatory effect |
|
Antirheumatic biologicals |
|
Apo A-IMilano* |
|
Delipidated HDL* |
|
Bariatric Surgery; RYGB |
|
Promote proinflammatory HDL |
|
Atherosclerotic CAD |
|
Diabetes mellitus |
|
Chronic Haemodialysis |
|
A diet high in saturated fat |
|
Infections |
|
Rheumatoid arthritis |
|
Surgery |
|
Systemic lupus erythematosus |
Table 2 Factors that can make the “good “cholesterol better, or bad, *Currently in Development
Permission of: (modified) from Ansell BJ, Fonarow GC, Navab 2007;9:57–63. with permission from current medicine group, LLC, Philadelphia, 2007.
New Tests for HDL Assessment Functionality
It is increasingly evident that the functional attribute of HDL holds comparable or greater significance than the quantitative HDL level (Figure 13).
Figure 13 Permission of: LFA1 denotes Lipid Free Apo Lipoprotein A1 (Source: Tziakas D, Chalikias GK. (2007). Role of apolipoprotein E genotype in coronary artery disease. Future Cardiology. 3(5):537–551).
Under normal circumstances, HDL promotes RCT from arterial walls to the liver, slows vascular inflammation, and limits oxidation of LDL particles, all of which help keep plaque formation under control. However, in the setting of systemic inflammation, HDL may show impaired function in these same biological capacities. Moreover, evidence suggested that HDL may occasionally have a conflicting role; by enhancing oxidative and inflammatory pathways that lead to the development and progression of atherosclerotic plaques.45
Several in vivo and in vitro analyses have been developed to assess HDL’s various functions. These tests are not currently available for commercial use but show efficacy in the assessment of the functional capacities of the patient's HDL beyond quantitative estimates of the HDL-C.
Monocyte chemotaxis analysis
This test measures the affinity of serum monocytes to be attracted to in-vitro vascular endothelial cells towards LDL before and after HDL is added. Within the human body, the arterial endothelium shows an enhanced production of MCP-1 when exposed to LDL. This augmented production leads to accelerated attraction of monocytes towards the arterial wall, consequently enforcing inflammation within the arterial wall.
In normal healthy circumstances, HDL significantly reduces the production of MCP-1, leading to a decrease in the monocyte migration towards inflammatory sites. Whereby HDL has the full potential of anti-inflammation. If HDL shows an increased production of MCP-1, it is considered pro-inflammatory. The effect of HDL on monocyte chemotaxis shows an inverse relation with HDL's RCT activity.
Adhesion molecules
The effect of HDL on the expression of adhesion molecules on the surface of endothelial cells can be measured. Workers on this modality reported that levels of endothelial cell intercellular ICAM-1 and VCAM-1 may be utilized as signs for evaluation of the effect of HDL on the cell’s inflammatory receptors. Fewer adhesion molecules indicate that the patient's HDL has anti-inflammatory properties, while increased levels indicate pro-inflammatory properties.46,47
Cell-free assay: Navab et al. developed this test to assess the effect of many antioxidant enzymes within HDL particles toward oxidation of phospholipids, which are the main constituents of both LDL and HDL.48–50
Copper stimulation: Hasselwander et al. worked on a different technique to assess HDL’s effect on the production of oxidized lipids in response to copper stimulation, the reaction might differ from oxidative stress that occurs in the body.51
The suggested future laboratory tests for the measurement techniques and biomarkers of cardiovascular risks; are in 3 categories (source Below)
LCAT assay, HDL-C efflux assay, non-radioactive assay for cholesterol exchange onto lipid poor apoA-1, HDL anti-inflammatory assay52,53
Source: BBA Clinical: Review HDL: Measurements Techniques and Potential biomarkers of Cardiovascular risk 2015 Published by Elsevier
The two faces of HDL ansell
Effects of Medications on HDL -C level and functionality
Statins
Statins are the compounds that act as inhibitors of HMG-CoA reductase, a major enzyme involved in the last steps of cholesterol synthesis. They are reputed to be very effective therapeutics in lowering LDL-C, besides their modest effect on raising HDL-C. Variability is observed throughout the dose level and individual statin that has been used. The increased HDL-C is guaranteed with specified doses in some statins, such as simvastatin and rosuvastatin. Other statins might not give the same dose-dependent result. In the case of atorvastatin, the maximum elevation in HDL-C levels is obtained at the lowest dosage used. The positive alteration in HDL-C is specifically associated with increased apo A-I levels as demonstrated by most of the studies conducted in this regard.25
Of note, it had been reported that there is no correlation between the drop in LDL-C and the rise in HDL-C levels. Both are independent of each other. Moreover, the mechanism by which statins increase HDL-C and apo A-I levels remains to be explained and is not related to their main action which is the inhibition of the HMG-CoA (Pleotropic effect).
The ability of statins to enhance hepatic ABCA1 gene expression, besides the proven statin-induced suppression of CETP, may shed light on the effect of stain on HDL-C levels.
Statins have also shown the ability to modify HDL pro and anti-inflammatory properties.
The study of simvastatin 40 mg for 6 weeks. In patients with established CAD or risk equivalents, their HDL altered from pro-inflammatory to more anti-inflammatory, as tested by the most relevant tests: the monocyte chemotaxis assay and the cell-free assay.
Charles-Schoeman et a. showed that HDL from patients with active rheumatoid arthritis became more anti-inflammatory after the patients were treated with atorvastatin 80 mg daily for 12 weeks (Table 3).54,55
|
Trial |
Benefit |
|
AIM-HIGHa |
No benefit of niacin |
|
HPS2-THRIVEb |
No benefit of niacin/laripoprant |
|
Mendelian geneticsc |
No difference in CV risk with HDL variants |
|
ILLUMINATEd |
No benefit (harm) of torcetrapib |
|
dal-OUTCOMESe |
No benefit of dalcetrapib |
Table 3 Permission of a. AIM-HIGH Investigators. N Engl J Med. 2011; 365:2255-22677; b. HPS2-THRIVE Collaborative Group. N Engl J Med. 2014; 371:203-2128. c. Voight BF, et al. Lancet. 2012;10 d. Barter PJ, et al. N Engl J Med. 2007;357:2109-212210; e. Schw artz GG, et al. N Engl J Med. 2012;367:2089-2099
Fibrates
Fibrates are synthetic ligands for PPAR-α, significantly lower plasma triglyceride levels (30–50 %) with a modest rise in HDL-C levels (5–15 %), most of the documented studies hadn’t shown major differences across the compounds available.
Stimulation of PPAR-α ligands in the liver leads to β-oxidation of free fatty acids therefore reducing VLDL biosynthesis and secretion. In addition, there is increased expression of the lipoprotein lipase gene, on the other hand, there is decreased expression of apolipoprotein C-III. As a result, there is an enhanced hydrolysis of lipoproteins rich in triglycerides. HDL-C is elevated due to the augmented expression of apo A-II and, to a lesser extent; apo A-I. Moreover, this increase is attributed to the efficient cholesterol disposal by the activation of ABCA1 in the liver. Data obtained from studies have shown that fenofibrate induces a reduction in the hepatic expression of CETP.38
Niacin
Niacin (nicotinic acid), a GPR109α agonist administered in therapeutic practice for over five decades to reduce cholesterol and triglycerides, increases HDL-C levels might be up to 30%. All the different niacin formulations (regular and extended release) are equally effective in increasing HDL-C levels, whereas acipimox (a nicotinic acid analog) induces the effect of a 7 % increase. The adverse effects of niacin are of special concern, which include flushing, gastrointestinal upset, alteration in liver function tests, and worsening glucose control. The use of the prostaglandin receptor antagonist laropiprant has been observed to significantly improve but not entirely the flushing and skin symptoms. The mechanism of niacin increase in HDL-C levels remains unproven and to be explained. Many authors have hypothesized Niacin’s ability to restrain CETP inhibition, stimulate ABCA1 expression, and decrease HDL breakdown.38
Therapeutics and CVD
Currently, Statin therapy has been reputed to be effective for risk reduction in atherosclerotic CVD and improved mortality. Their major impact is attributed directly to the reduction in LDL-C levels. Residual risk factors should be taken into consideration to achieve maximal care for the escalating number of atherosclerotic CVDs. The utmost importance of that factor is to increase HDL-C levels. Increasing HDL-C levels has been observed as a vital and rewarding approach for the improvement of CV mortality.
However, the data of the major trials are challenging regarding raising the HDL-C levels, on the other hand, it has been documented that fibrates have a remarkable effect on raising HDL-C levels, but the reports are conflicting regarding their benefit on CVD endpoints.25,39
In the FIELD study that involved T2DM patients, there was a significant reduction in CV events, among the cohort of low HDL-C level while the cohort group with high HDL had demonstrated minimal CV benefit.
In the ACCORD trial, it demonstrated no significant benefit from the use of fibrates to statins. The recent trials that had been conducted incorporating niacin with statin had not demonstrated significant effectiveness and appreciable positive CV outcomes, in contrast to old conventional trials that declared some benefits. Unfortunately, most CETP inhibitors that have been introduced, despite the noticeable potential of effectively raising HDL-C levels, have not exhibited positive results in favor of CV risks. On the other hand, the HDL mimetics represent the more promising therapeutic potential for improving the CV outcome. However, their application in current clinical practice mandates more data and investigations on morbidity and mortality.
Future therapeutics
The inclusion of this compound in specified patients with acute coronary syndrome resulted in a remarkable reduction in atheroma size (mentioned earlier). However, subsequent obstacles and challenges in manufacturing, as well as the most recent pilot trial with the most refined product did not have favorable outcomes towards CAD progression. The introduction of this therapeutic potential was curbed by commercial cost for wide-scale use and multiple intravenous settings.31,40
Technically selective dilapidation could convert α HDL to pre β HDL, resulting in more effective lipid scavenging activity of new HDL, Autologous delipidated HDL when infused with patients with acute coronary syndrome be well tolerated and vital, atheroma volumes had shown a decrease in volume in comparison to increase in control group. More data are required to show more confirmed statistical differences.
The trial (AEGIS Trial) was shown by introducing 4 weekly infusions of CSL112, which was well tolerated and relatively free from biochemical imbalances, for patients with acute myocardial infarction. The CTE capacity was confirmed. In phase 3, the objective was to evaluate the therapeutic efficacy and benefit in improving major CV events (32).
Alternative Pathway
If we review the classical book teaching or literature about RCT we will find that the transport of cholesterol from the peripheral tissues to apo A-I of HDL-C through a process which is carried on by ABCA1, ABCG1, and/or ABCG4 pathways. Then the carried cholesterol will be transported to the liver by SR-BI. The next step is hepatobiliary secretion which is mediated by ABCG5/G8 which facilitates final excretion through the bowel (Figure 14).
This concept has been taught to us for many decades, but there are accumulated pieces of evidence that have come out from new research that directed our attention towards debating this concept as it will not give answers to many questions. Therefore, it ought to be reviewed and an alternative pathway (for cholesterol disposal TICE) should be considered as shown briefly in the schematic figure how non-biliary cholesterol reaches the intestine, in this alternative route is a matter of future extensive debate.
Recent research in the last decade, by using new techniques and specified mouse models. Interesting results were filtered, that immediate transintestinal excretion of plasma cholesterol might contribute to reverse cholesterol transport. Based on the results from animal studies, it is estimated that this non-biliary route may account for around 30% of total fecal neutral sterol excretion under basal conditions and could be modulated by liver X receptor (LXR), peroxisome proliferator-activated receptor-delta (PPAR-d), and farnesoid X receptor (FXR).56
Moreover, there are solid pieces of evidence from animal studies that suggest that this pathway might represent a novel therapeutic potential to increase RCT and subsequently offer cardiovascular protection.
More recently, the contribution of TICE to total fecal-neutral sterol excretion is investigated in humans, utilizing a cholesterol balance approach with combined stable isotopes with labeled cholesterol and bile acid molecules, 15 men with mild hypercholesteremia were given a cholesterol absorption inhibitor (10 mg Ezetimibe) for 4 weeks, and the body cholesterol efflux was determined, on the other hand, the same study was carried out on 10 individuals consuming a regular meal.57–60 Under standard conditions, approximately 65% of daily fecal neutral sterol excretion originated from biliary secretion and the remainder (~35%) is more likely derived from TICE.53,61–70
According to the previous elaboration of clinical, and epidemiological data, it has been shown by solid evidence that the functionality of HDL exerts a major and critical role in combating the risk and progression of atherosclerotic CVD protecting against more than merely quantitative levels of HDL-C.
HDL-C involves a heterogeneous group of particles that exhibit variable structure and many biological functions. It is noteworthy that pronounced high serum levels of HDL-C have not steadily exerted absolute protection against atherosclerotic CVD, as a matter of fact in some situations may potentially enhance inflammation throughout the body.
The HDL-C functionality is conditioned by various elements; hereditary, environmental, and lifestyle modifications. HDL -C CEC is the pivotal function that addresses the crucial functionality of HDL in improving CIMIT and atherosclerotic CAD.
Therefore, regardless of HDL-C level, thorough medical research, investigations, and data interpretations are running to isolate the definitive therapeutic potentials that offer safe, effective profiles. These presumed agents would have to promote CEC and improve HDL-positive functions thereby consequently reducing the risk of atherosclerotic CVD.47,48
WHAT is imperative for practicing clinicians?
Although Low levels of HDL-C can be utilized as a clinical indicator of CHD risk, raising HDL-C levels would not necessarily eliminate this risk. This is attributed to the complex properties of the HDL-C particle (or particles). Clinical situations that trigger systemic inflammation, like systemic sepsis, autoimmune and rheumatological disorders, T2DM, and pre-existing atherosclerosis enhance the proinflammatory potential of HDL as well. However, some anti-atherogenic measures might promote the anti-inflammatory potential of HDL-C.
As far as the efficient analyses for HDL functionality are in progress, the therapeutic pool to improve HDL’s anti-inflammatory (and probably other functions) is in development and the ongoing investigations into the lipid-modifying therapy and lifestyle alterations would enhance or inhibit HDL function. Furthermore, studies are ongoing to correlate HDL’s proinflammatory aspects to testing of HDL sub-fractionation.
Presently, laboratory testing of HDL’s anti-inflammatory capacity will permit the clinicians to know the specified patients who are eligible for the qualitative function of HDL therapy (Figure 15, a & b zoom in)
Invention and evaluation of future therapies may be helped by assessing their effect on HDL function.
Therefore, we are still waiting for the therapeutic interventions and analytic tools to develop and to be put under hands in clinical practice, so practicing physicians may be worthy to consider the following:
Lastly the “HDL-Cholesterol story” is not accomplished, nevertheless, the recent findings open the avenues for future research on this complex lipoprotein.
Mr. Ammar S Al Jadir, BSc, SEIMENS ENERGY, Baghdad, IRAQ; for assistance in preparing the electronic manuscript.
Authors declare that there is no conflict of interest exists.
None.

©2023 Jadir. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.