eISSN: 2473-0815


Mini Review Volume 5 Issue 4
University of Birmingham, UK
Correspondence: Dominic Worku, University of Birmingham, 14 cwrt ucha terrace Port talbot SA13 1LD, UK, Tel 07780663345
Received: July 24, 2017 | Published: September 8, 2017
Citation: Worku D. The future of steroid therapy in inflammatory disease. Endocrinol Metab Int J. 2017;5(4):249-253. DOI: 10.15406/emij.2017.05.00127
steroid therapy, inflammatory disease, rheumatoid disease
HPA, hypothalamic-pituitary-adrenal; CRH, corticotrophin releasing hormone; 11β-HSD, 11β-hydroxysteroid dehydrogenase; EULAR, european league against rheumatism; GREs, glucocorticoid response elements; NLS, nuclear localization sequence
Glucocorticoids/ ‘steroids’ are amongst the most widely prescribed drugs in medicine with a global market worth of >$10 billion/year and considerable patient awareness1,2 GCs were first discovered as the adrenal hormone capable of fully reversing the pathology of Addison’s disease.3 It was Philip Hench (1948) however who first noticed that female patients with rheumatoid disease showed clinical improvements when pregnant, this led to the search for some adrenal factor responsible and the ensuing discovery of the primary endogenous glucocorticoid, cortisone (inactive)/ cortisol (active). Upon introduction of synthetic formulation of cortisone to women with arthritis significant pain relief and mobility was obtained within 3 days of treatment and was heralded as a miracle.4 Since these humble beginnings several synthetic steroid preparations have been approved including dexamethasone and betamethasone which are particularly potent with dramatically less mineralocorticoid activity versus cortisone.4 Endogenous Glucocorticoids like cortisol are produced and controlled by the hypothalamic-pituitary-adrenal (HPA) axis. This is a key axis which regulates steroid hormone production in the body. Upon stimulation of the hypothalamus by some biological stressor (e.g. illness, fever, hypoglycaemia) as well as neural and cytokine inputs the periventricular nuclei of the hypothalamus of the brain releases Corticotrophin Releasing Hormone (CRH) which stimulates the anterior pituitary gland to release ACTH (adrenocorticotrophic hormone) which in turn stimulates the adrenal cortex to release glucocorticoids which through negative feedback reduce and inhibit the production of the aforementioned factors which stimulated its secretion(5).
Like most hormones of the body a 24 hour rhythm of normal secretion exists. For cortisol the circadian rhythm of its release is such that serum cortisol levels peak in the morning ~9am with a low at between 9-12pm. This morning surge can be understood to be preparing for the ensuing day and mobilising energy resources to switch from the sleep to the active state. Importantly however the changes in basal GC level do not occur evenly across the 24hr period with ultradian pulses of hormone release occurring.5
An important concept with regards to GC’s is the fact that a circulating pool of active steroid such as cortisol, corticosterone and prednisolone exist in a state of interconversion with their inactive counterparts (cortisone, 11-dehyrocorticosterone and prednisone respectively). This local control of circulating GC’s is achieved by 11β-hydroxysteroid dehydrogenase (11β-HSD) enzymes. While 11β-HSD1 controls the conversion from inert to active steroids (cortisone to cortisol) and is found primarily within the nervous system, liver, fat and lung; 11β-HSD2 catalyses the reverse and is found in the kidney, colon and placenta.6 Therefore the local concentration of GC’s acting at a given tissues, is controlled by not only the serum level of GC release by the HPA axis and thus the amount of free cortisol that is unbound from the plasma protein corticosteroid binding globulin (90% of all cortisol) but also the local tissue concentrations of the above enzymes.
The principle actions of synthetic GC’s are threefold and are identical to those of endogenous GC’s although they are varyingly more potent. Firstly, they serve to increase glucose mobilisation ensuring energy supply during times of biological stress. This is achieved by augmenting pathways such as increased fat metabolism and gluconeogenesis. Secondly they are important in mediating vascular tone and salt water balance and therefore maintain effective cardiac output and finally they modulate the immune response. It is for this last reason in particular that GCs are useful in medicine and the mechanism by which this is achieved is thought to be by several methods. These include the inhibition of key cytokines (e.g. IL-2), chemokines, inflammatory enzymes, MHC class II receptor expression and expression of cellular adhesion molecules. Due to these properties GC’s have found use in countless inflammatory disease from Rheumatoid arthritis, Asthma, Inflammatory Bowel Disease, brain oedema and transplantation.7
However while these drugs have been transformative for many conditions in most part by their systemic absorption, their use is plagued with unwanted side effects and indeed eventual resistance (Figure 1).8 GC resistance itself can either be acquired, genetic or part of the disease specific process with conditions like COPD, pulmonary fibrosis and Cystic Fibrosis being largely GC resistant. Indeed in those patients with genetic resistance to GCs they have high cortisol levels but without signs of Cushing’s syndrome and exhibit adrenal suppression in response to exogenous steroid. Microarray studies examining such patients have revealed 11 genes that are differentially expressed versus GC sensitive patients with evidence that cytokines including IL-2 and IL-4 have the ability to reduce GR nuclear translocation and binding affinity through a p38 MAPK dependent method.9 Such studies highlight the possibility of testing for GC sensitivity in patients. While examining polymorphisms of GR may hold the answer, examination of bone marrow monocytes has shown GR number, GRβ and MDR1 gene expression which encodes an important multidrug efflux pump to be key determinants of GC responsiveness.10
Compound |
In vivo results |
In vitro results |
RU-24858 |
Dissociative Profile |
Anti-inflammatory no improvement in side effect Profile |
RU-24782 |
Dissociative Profile |
|
RU-40066 |
Marked Transrepression |
Anti-inflammatory with improved side effect Profile |
AL-438 |
Preference for Transrepression |
Anti-inflammatory with improved side effect Profile |
ZK-216348 |
Preference for Transrepression |
Anti-inflammatory with improved side effect Profile |
Compound 25 |
Dissociative Profile |
Anti-inflammatory Activity |
Compound A |
Dissociative Profile |
Anti-inflammatory with improved side effect Profile |
Compound 6 |
------------ |
Antagonistic Profile |
Org 214007-0 |
Dissociative Profile |
Anti-inflammatory with improved side effect Profile |
Mapracorat (ZK-245186) |
Dissociative Profile |
Partial GC Agonist |
Fosdagrocorat |
Marked Transrepression |
Anti-inflammatory Efficacy in Phase 1 trial |
JTP-117968 |
Partial Transrepression |
No Activity on Mineralacorticoid receptor |
Table 1 List of known SGRMs/SERMS and their profile of action19-23
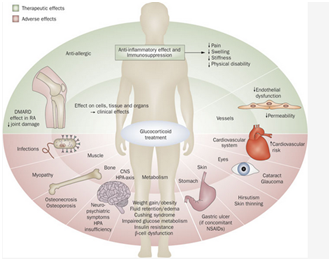
Figure 1 Glucocorticoid mediated effects in the body both favourable and adverse.7
As mentioned before these viewed side effects which are incorrectly named are not inconceivable but are instead clearly related to the physiological functions of GCs. Indeed the risk of these perceived side effects are proportional to the cumulative dose of GC given as it does the mode of steroid delivery with local preparations (topical and intra-articular) offering the least side effects. Interestingly, we find divergent views regarding their perception of the occurrence and severity of GC related side effects some of which are irreversible. While patients are concerned more with the fatigue, depression and aesthetic side effects clinicians are more worried about their long-term manifestations including delayed wound healing, infection, short stature, hypertension through sodium retention, osteoporosis, psychiatric disturbance, worsening glycaemic control and diabetes.11 Osteoporosis is indeed a major cause of morbidity in patients and is in fact the most common side effect of GCs as GC’s inhibit osteoblast function and differentiation resulting in poor bone density and strength. This increased fracture risk occurs at low dosages ~5mg Prednisolone daily and even with cessation of steroids, bone quality does not return to baseline.12 That being said current European League against Rheumatism (EULAR) guidelines suggest that the risk of harm is low ≤5 mg prednisone equivalent per day while doses between 5-10mg dependent on patient specific factors.13
As a result of these side effects the use of GC’s is often met with caution and long term use is discouraged. So is it possible to produce a synthetic steroid that would allow for dissociation of their benefits and perceived side effects. To understand if this is possible we must first look at how GCs produce their genomic effects. Due to the underlying lipophilic and hydrophobic structure of steroid hormones they can infiltrate the cell membrane and upon doing so can bind to cytoplasmic GRs which are ubiquitously expressed and modular in structure and most of the time bound by chaperone proteins (e.g. hsp90) and immunophilins (e.g. FKBP52) in an inactive state.14
The GR found in every cell of the body except red blood cells is composed of 3 functional units: N-terminal transactivation domain, central DNA binding domain and C-terminal ligand binding domain. Upon ligand binding to the GR, conformational changes allow for the dissociation of chaperone proteins, GR monomer dimerization to occur and exposure of the nuclear localization sequence (NLS) for subsequent translocation of the ligand receptor complex to the nucleus along the microtubular cytoskeleton. Upon reaching the nucleus, the GR-ligand complex is translocated into the nucleus through nuclear import proteins (e.g. importin α) where the GR functions as a transcription factor binding to DNA at key palindromic DNA sequences of genes termed Glucocorticoid response Elements (GREs). Through this binding and the recruitment of coactivators such as cAMP response element binding protein which has intrinsic histone acetyltransferase activity allowing for the recruitment of remodelling proteins and subsequent RNA polymerase II binding to initiate gene transcription. It is currently thought that 10-100 genes of each cell type are directly regulated by GCs by this method.14,15 A key point in this process is that binding of GC bound GR to these GRE DNA sequences where activation and expression of genes is the response is achieved in GR dimers. This is called Transactivation. Conversely, inhibition of gene expression can be produced by the GC bound GR monomers binding to pro-inflammatory transcription factors (e.g. nF-kB, AP-1) already bound to DNA and effectively switching them off and inhibiting their target genes expression. This is the tethered indirect mechanism of Transrepression (Figure 2).16 The direct method of Transrepression implicates inverted repeated negative response elements and involves GR SUMOylation (SUMO-Small Ubiquitin related modifier) in the N-terminal domain of the GR and the formation of associated NCoR1-HDAC3 complexes. However, it is by far the tethered mechanism of transrepression which is most important and occurs due to interference with the phosphorylation of RNA polymerase II. Current opinion is that transactivation and direct transrepression are responsible for the majority of GC induced side effects.17
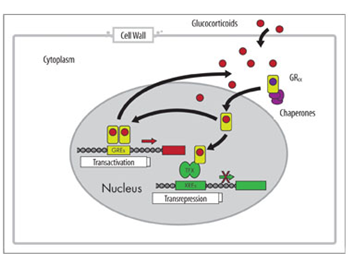
Figure 2 Transactivation and Transrepression Mechanism of Glucocorticoid genomic action.16
To discover how the GR works and the methods by which side effects may be avoided researchers had to create suitable animal models. In the first mice models which were GRnull, offspring were produced with severe lung abnormalities and early death, highlighting the importance of GR in development. As such a new GRdim/dim model was made. The mutation in this model affects GR homodimerization and thus impairs transcription of related GREs. These mice are however viable and maintain physiological GR mediated functions including their anti-inflammatory actions although are more susceptible to inflammation.12
As such if we could produce synthetic steroids that transrepress then we could produce all their beneficial inhibitory anti-inflammatory functions without the positive upregulation of genes involved in their vast side effect profile. Such an arrangement is termed a dissociative profile of action. While idealistically easy this remains difficult to attain with the ability to separate these transcriptional events an important step in producing SEGRA’s/SGRM’s. It is now known that GR may be modified in many ways including phosphorylation, acetylation, sumolyation and ubiquitination. Of these GR modifications phosphorylation is the most frequent and is implicated in mediating GR ligand DNA binding affinity and subsequent GR cellular movement. This ultimately alters the dynamics of transrepression and transactivation with the phosphorylation of the S211 residue a hallmark of transactivation.18 However the current model of understanding whereby transactivation results in the side effects while transrepression mediates GC anti-inflammatory action is erroneous. Indeed transrepression is responsible for some side effects including HPA axis suppression while other side effects including osteoporosis are thought to be common to both pathways. However as mentioned is it thought that if we were able to produce transrepressive molecules that the vast majority of side effects could be avoided.
Initial screening of test SGRMs compounds has revealed several promising candidates (CpdA, AL-438, ZK-216348, and RU-24858) which have shown the ability to both bind to GRE’s and nF-KB binding sites when pro-inflammatory stimuli were introduced (Table 1).19 Of these Compound A (CpdA) was the earliest to be identified and was first isolated from the Namibian shrub (Salsola tuberculatiformis). This non-steroidal compound when investigated was found to bind atypically to the GR in ligand binding essays but with high affinity, and was thought it may be using different coupling points in the Ligand binding domain of the GR or by inducing a unique conformation of the GR which is not yet understood.20-24
Indeed this compound can at doses of 10-3M transrepress nF-KB production which has corresponded to disease modifying properties in a collagen type II induced RA mice model without major side effects and has not been implicated in eventual GC resistance. In addition this compound is widely studied in in vitro and in vivo studies however the narrow therapeutic index of this compound alongside its structural malleability limits its use in the real world but does make it an effective platform to research the concept. For instance in an animal model of Multiple Sclerosis (MS), CpdA showed repression of myelin specific T-effector cells through downregulation of cell adhesion molecules but was noted to cause apoptosis in several cell lines including lymphocytes with weight loss a common side effect(24). In addition in an inflammatory bowel disease study both CpdA and ZK216348 had wound healing properties by positively upregulating intestinal cell proliferation and migration. This is something that first demonstrated in GRdim mice that exhibited improved wound healing versus wildtype controls through a supposed TGFβ mediated mechanism.25
While the genomic pathway of steroid action is understood, the non-genomic aspect of their action is yet to be fully explored. Evidence that GCs must have non-genomic action is based on the fact that GCs have effects within minutes of administration. Several classifications of non-genomic action of steroids exist although the Buttgereit classification is the easiest to appreciate. Firstly, steroids can have as yet poorly defined interactions with the cellular membrane whereby they can influence the properties of membrane bound proteins. Secondly, GCs can mediate non-genomic effects through cytosolic GRs and their bound chaperone proteins which as part of the GC-GR binding process are uncoupled and free to interact with other cytosolic proteins. Thirdly, GCs may interact with membrane associated GRs (mGRs) which are thought to be involved in intracellular signalling processes with over 51 kinases measured to have increased activity after mGR activation. Indeed through mGR activation several cellular processes are thought to be affected including electrolyte levels, mitochondrial reactive oxygen species production and lysosomal enzyme release with some sources stating that these receptors are actually a GRα subtype.26 Currently, it is not known what effect if any SERMS have on this pathway of steroid action. Initial reports concerning this have shown that CpdA can block all Mitogen Associated Protein Kinases (MAPKs) of fibroblasts in a rheumatoid arthritis model.12
Due to improvements in the understanding of GR structure researchers have been able to design better SERMs with improved functionality; one example of this is Org 214007-0.21 This was based on the fact that this partial GC agonist it can interact between helix 11 and 12 of the ligand binding domain of the GR a point where co-activator binding takes place at a similar affinity to prednisolone with a maintained in vitro profile of action in several studied cell lines. In addition it was found that this compound did not shift the balance of liver glucose/glycogen balance and did not predispose to hyperglycaemia and retained its anti-inflammatory activity in a CIA mouse model at a similar efficacy to prednisolone. This is an important consideration has GC induced muscle atrophy is a big problem and culminates in a Cushings phenotype. Gene’s upregulated as part of this process include MuRF1 which was found not to be upregulated by Org 214007-0 treatment. As such this is a very interesting compound which can be used as a platform to study future SERMs and is being suggested for phase 1 clinical trials in an MS model.21
In comparison LGD-5552 is a newly identified nonsteroidal benzylidene compound able to competitively bind to and repress the GR as demonstrated by strong antagonism of dexamethasone induced luciferase activity and inhibition of E-selectin and IL-6 promoter. Interestingly while maintaining weak mineralocorticoid activity this compound was able to upregulate transcription of anti-inflammatory cytokine IL-10 by up to three fold which is known to reduce Lipopolysaccharide induced gene expression when compared to prednisolone in both acute and chronic models of inflammation. These in vivo results within a rodent model of EAE (autoimmune encephalitis) it was found that LGD-5552 at doses of 1-3mg/kg had no effect on mean arterial blood pressure (MABP) but produced even greater efficacy than prednisolone and completely ameliorated disease (mean disease score=0) with no signs of motor impairment (p=0.0002) and no changes in body weight. As such going forward this remains a safer alternative to current GC based treatment in models of disease.20
Before we can suggest however that these compounds have a role in replacing current GC treatment we must confirm that SGRMs have a role within multiple models of inflammation. An important condition in which steroids are used is in inflammatory bowel disease and may be required routinely to control disease. Investigations of GR agonists CpdA and ZK-216348 in acute colitis have demonstrated that it can ameliorate the signs and symptoms of the disease while limiting the occurrence of severe attacks. This is an important consideration as the GC is retaining their disease modifying properties without the risk of sudden withdrawal or tailored reduction. Interestingly, ZK-216348 demonstrated a dose dependent response while CpdA showed limited response above dosages of 10mg/kg this would suggest that between related steroidal compounds that the pharmacokinetics and pharmacodynamics need to be explored.25 In the one animal model studying the effects of the dissociative steroid ZK-245186 which has already demonstrated improved safety profile in rodent models of dermatitis. In vitro studies by the author in this model showed that ZK-245186 could inhibit TNFa secretion from Peripheral Blood Mononuclear Cells (PBMC) and Bone Marrow Dendritic Cells (BMDC). Indeed after 14 days of use ZK-245186 did not impact skin fold thickness but did lead to significant improvements in erythema and wheals appearance.27 It is of no surprise therefore that this compound was trialled in a human model of atopic dermatitis with the results yet to be reported.28
Current research has indicated how SGRMs may be able to improve anti-inflammatory activity in disease states through methods other than affecting nF-kB binding to DNA. Current understanding of nF-kB signalling is that two main pathways of action exist the classical and alternative pathway. While the former of these is known to when dysregulated be involved in disease processes such as cancer, T2DM and autoimmune disease the latter has been shown to negatively regulate inflammation with RelB a key protein in this pathway. Mapracorat is a novel SEGRA being investigated in both dermatological and ocular conditions. Indeed in the latter of regards this compound is thought to be as effective as dexamethasone but with an improved safety profile. It was found within human ocular cells that this compound was able to stabilise and upregulate RelB levels for up to 24h after exposure and limit COX2 activity leading to sustained improvements in the inflammatory profile in in vivo studies. This suggests that SEGRAs have many more downstream effects than first thought and increasingly begs the question of how they mediate their effects something which current research is trying to understand which we need to in order to predict likely side effects. However whether SEGRA mechanism of actions is cell line dependent remains to be seen.29
However, as the quote at the beginning of this piece states, all is not as it seems. In fact there have been lots of cases where dissociated GR ligands in vivo have produced classical side effects with less anti-inflammatory action than classical GC’s such as prednisolone and may reflect that the divergence between transrepression and transactivation profiles is over simplistic for as mentioned previously these are all physiological GC effects. Moreover, growing evidence suggests that some of the inhibitory gene and anti-inflammatory actions of GCs have been incorrectly assigned to the transrepression mechanism of GC use and may in fact be due to transactivation of genes. In fact the discovery of non-palindromic GRE’s to which GR dimers can bind and produce negative expression of genes suggesting multiple overlapping pathways of gene expression yet to be explored. Furthermore, it has proven almost impossible to predict the type of transcriptional response from the sequence of the binding site and as such it could be the local concentration of coactivators and corepressors directing whether at a particular gene GRE interface is inhibitory or stimulatory in nature and not whether a GR monomer or dimer is bound.
It seems a better understanding of GC-GR-GRE interactions is required if the pursuit of dissociated ligands is likely to be fruitful. That being said there are many promising compounds which have demonstrated good in vivo activity in models of acute and chronic inflammation. However before any progress can be made we must be able to confirm the longevity of the genetic antagonism of transactivation. As such these compounds may soon have an important place in patient care and may one day come with the first risk free guarantee with human studies clearly the next step in the pursuit of this goal.

©2017 Worku. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.