eISSN: 2473-0815


Review Article Volume 4 Issue 6
1Rheumatology Department, Cady Ayyad University, Morocco
2Anesthesia and Resuscitation Department, Mohammed V University, Morocco
3Rheumatology Department, Cady Ayyad University, Morocco
Correspondence: Imad Ghozlani, Rheumatology Department, Military Medico Surgical Center, Cady Ayyad University, PO Box, 4024, Morocco, Tel +212(0)661590176
Received: March 14, 2017 | Published: June 14, 2017
Citation: Ghozlani I, Filali KE, Niamane R. Rheumatoid cachexia, a metabolic rheumatoid arthritis disorder: pathophysiological mechanisms, diagnostic tools and current therapeutic strategy. Endocrinol Metab Int J. 2017;4(6):149?155. DOI: 10.15406/emij.2017.04.00106
Rheumatoid arthritis (RA) is a debilitating, chronic, autoimmune and inflammatory disease in which pro-inflammatory cytokines play a crucial role. It is characterized by joint stiffness, pain, and swelling, and is accompanied by a loss of body cell mass known as rheumatoid cachexia (RC). It is a complex metabolic syndrome associated with underlying illness and characterized by loss of muscle with or without loss of fat mass. The exact pathophysiological mechanisms underlying the development of RC are not fully understood, but multifactorial mechanisms have been investigated. Its include excessive cytokine production, physical inactivity, and reduced peripheral insulin action. Several techniques such as magnetic resonance imaging (MRI) and dual-energy X-ray absorptiometry (DXA) will help define with more confidence the mass and distribution of fat and muscle and help elucidate the relationships between body composition and outcomes. Rheumatoid cachexia may be an important risk factor for cardiovascular disease, osteoporosis and excess mortality in RA. In this context, despite great advances in the treatment of RA, there is a lot more evidence in favour of nutritional interventions and physical activity prescription for patients with RA.
Keywords: rheumatoid arthritis; rheumatoid cachexia; diagnostic tools; nutritional management; physical activity
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease of unknown etiology that is associated with reduced life expectancy and causes destruction of joint cartilage and bone.1,2 It affects nearly 1% of the population and predominates among women.3 The nutritional impact of RA is associated with inflammatory diseases and comprises the effects of nutritional alterations on motor and sensory functions, manifested by a combination of weakness, muscle atrophy and loss of functionality.4,5 Many studies have identified changes in body composition as contributing to the increased morbidity, as well as the mortality, associated with RA.6 Hypermetabolism and protein degradation linked to the pro inflammatory cytokines induced by the disease cause reduction of fat-free mass (FFM), often associated with increased fat mass (FM) and thus with little or no weight loss, and a maintained body mass index (BMI).
This combined condition has been called “rheumatoid cachexia” (RC).7 In recent decades, the concept of rheumatoid cachexia has received more consideration5 based on the understanding of how nutritional alterations affect patient quality of life, evolution and the prognosis of rheumatologic conditions such us increased risk of cardiovascular disease and osteoporosis.8,9 Advances have also been made in our comprehension of the pathophysiological mechanisms that mediate the nutritional impact of inflammatory diseases (acute and chronic).10,11 Mean while, the tools available for evaluating and staging RC have become more precise and can more adequately define the effects of inflammatory conditions on lean mass, fat mass and energy expenditure.12
Epidemiology data
Because no consensual definition of cachexia exists, the prevalence of malnutrition, including RC, in RA varies with definitions, methods, and populations, and is reported to range between 26% and 71%.8 In Moroccan patients with diagnosed rheumatoid arthritis (RA), 53.9% of patients had RC.9 It is therefore evident that the prevalence of malnutrition, according to the criterion of decreased lean mass, is frequently underestimated. In Mexican patients with diagnosed rheumatoid arthritis (RA), 13 48% of patients had decreased lean body mass, even when 94% had increased fat mass percentages. It is therefore evident that the prevalence of malnutrition, according to the criterion of decreased lean mass, is frequently underestimated.5 The prevalence of RC also varies depending on the gender of the population studied: in patients with RA, it can reach 52% in women and 30% in men.13
Physiopathology of rheumatoid cachexia
The exact pathophysiological mechanisms underlying the development of RC are not fully understood. But several potential mechanisms have been investigated. The etiology is probably multifactorial. It includes excessive cytokine production, physical inactivity, and reduced peripheral insulin action. The inflammatory cytokines tumor necrosis factor α (TNFα) and interleukin 1ß (IL-1ß) are thought to be centrally involved in the pathogenesis of RA.14 Both cytokines are produced primarily by monocytes and macrophages, but they are also produced by a variety of other cells including B lymphocytes, T lymphocytes, and skeletal muscle.15
Concentrations of pro-inflammatory cytokines are high in patients with active RA; these substances act by stimulating the release of tissue-destroying matrix metalloproteinases as well as by inhibiting the production of endogenous inhibitors of these metalloproteinases, the net result being joint damage. Not only are TNFα and IL-1ß centrally involved in causing joint damage in RA, but these cytokines also exert a powerful influence on whole-body protein and energy metabolism. The so-called sarcoactive “muscle-active” cytokines include IL-6, interferon-γ, and transforming growth factor-ß, in addition to TNFα and IL-1ß. Although the specific mechanism by which TNFα and IL-1ß exert their catabolic effect is not known, it has been shown that subjects with RA have higher rates of whole-body protein breakdown compared with young and elderly healthy subjects, and TNFα is thought to stimulate muscle catabolism.16
It has been demonstrated that patients with RA have low physical activity. Many factors contribute to reduced physical activity among patients with RA, including joint pain and stiffness, metabolic changes leading to loss of muscle mass and strength, and simple disuse, perhaps related to general caution regarding physical activity.17 Physical inactivity is associated with changes in normal physiological processes leading to muscle atrophy and insulin resistance.18,19\ Lack of effective muscle stimuli decreases the turnover rates of muscle and whole-body proteins with inhibition of protein synthesis (Figure 1).20 These factors also lead to the initiation and progression of RC.21
The growth hormone (GH)/insulin-like growth factor (IGF) axis may also be an important contributor, although controversy exists.22,23 In an animal model of chronic inflammatory arthritis, Ibanez de Caceres et al.24 reported a decrease in body weight (BW) and reduced levels of circulating IGF-1; administration of recombinant GH was associated with increased BW gain without an increase in food intake, and increased levels of IGF-1. On the other hand, in the model, arthritis was also associated with increased expression of the cyclo oxygenase (COX)-2 gene; and administration of non-steroidal anti-inflammatory drugs (NSAIDS) reversed the COX’s inhibitory effect of arthritis on BW, increased liver IGF-1 levels, and enhanced the expression of ubiquitin-ligating enzymes.24 The authors suggested that COX-2 expression was responsible for the arthritis-induced cachexia, through modification of the GH-IGF axis and the ubiquitin-proteasome pathways.25 The use of recombinant GH in cachectic humans has been also approved for use in patients with AIDS,26 however, its efficacy in patients with RA is unclear.
The cachexia score (CASCO)
In spite of the existence of different cachexia definitions and consensus, cachexia is infrequently diagnosed. This is, in part, due to the lack of clear standardized cachectic markers. Some of the criteria used in the past include weight loss, decreased physical performance, fatigue, anorexia, and metabolic alterations. The group of investigators who met in Washington27 concluded that cachexia can be diagnosed in the following way: a weight loss of at least 5% or more in 12 months or less in the presence of underlying illness, plus three of the following criteria: decreased muscle strength, fatigue, anorexia, low fat-free mass index, abnormal biochemistry (increased inflammatory markers [C-reactive protein>5.0mg/l), IL-6>4.0pg/ml), anemia (<12g/dl), and low serum albumin (<3.2g/dl)].27 However, other diagnostic tools should not be discarded, such as decreased physical performance (total activity, handgrip strength, stairs climb, or 6-min walk distance) or biochemical tissue analysis (activation of proteolysis or apoptosis in skeletal muscle biopsies).28
The cachexia score as already stated above, in addition to clear and objective diagnostic criteria, an essential requirement for both clinical trials and patient treatment is a staging system that allows for classification of the cancer patients according to the severity of the cachectic syndrome. Such a staging system would be beneficial not only for assessing the severity of the syndrome but also to decide the type of treatment. It was the aim of the present study to develop such a classification system. The result is the newborn CASCO which, by means of a numerical scale, classifies cachexia into mild (0-25), moderate (26-50), severe (51-75), and terminal (76-100). It is therefore clear that the higher the score, the worst the syndrome (Figure 2).28
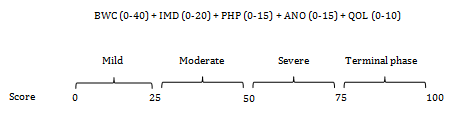
Figure 2 Cachexia Score (CASCO) staging scale.
BWC: body weight loss and composition, IMD: inflammation/metabolic disturbances/immunosupression, PH: physical performance, ANO: anorexia, QO: quality of life
Dual-energy X-ray absorptiometry (DXA)
DXA relies on the fact that an X-ray beam passing through a complex substance (e.g. the body) is attenuated to a different extent by the different substances it passes through.29,30 Using a dual beam with different X-ray images the machine is able to partition the body into two compartments on a pixel-by-pixel basis. Dense pixels corresponding to the bone are separated from less dense soft-tissue pixels (Figure 3). The soft-tissue compartment is then analysed to separate fat mass from lean body mass (LBM),31 DXA has emerged as the most clinically useful technique for measuring bone and soft-tissue mass.32 This method has also not been independently validated.33
Whole-body magnetic resonance imaging (MRI)
MRI provide a good approximation to anatomical measurements of skeletal muscle (SM) and fat, allowing visceral adipose tissue (VAT) to be distinguished from subcutaneous adipose tissue (SAT).34 They are expensive to use but approach the gold standard for measuring soft-tissue mass.35 In a typical protocol for whole-body MRI, the subject lies in a 1.5 T scanner in a prone position with arms extended above the head. Forty-one cross-sectional images are acquired, using a T1-weighted spin echo sequence, and subsequently analysed to determine volume of SM, VAT and SAT.35 Single-image slices through the mid-thigh and the mid-abdomen also provide excellent estimates of whole-body SM mass and fat mass, respectively and may provide a practical solution for clinicians and researchers.35 Whole body potassium counting and in vivo neutron activation for atomic analysis of the body are available in only a few centres world-wide. These techniques provide a unique window onto body composition as certain atoms are largely segregated into different tissue compartments such as bone (calcium), cells (potassium) and the protein ‘pool’ (nitrogen). The total body potassium content is taken to represent the body cell mass (BCM).36 This is calculated by measuring potassium-40, a naturally occurring isotope, which exists in a constant abundance of 0.0012%, using a body scintillation counter.37
Ultrasound
Muscle and subcutaneous fat thickness can be measured with ultrasound. A convenient area is the right quadriceps femoris muscle at the mid-portion between the greater trochanter and the lateral joint line of the knee.38 Ultrasound may be especially useful in the obese in whom anthropometric measures are inaccurate.39 Ultrasound mapping of muscle and fat thickness at different body regions and quantifying changes in topographical fat patterns are useful adjuncts to body composition assessment.39,40
Bioelectrical impedance analysis (BIA)
BIA utilizes the differing electrical conductivity of fat and lean tissue. The passage of an electrical signal is facilitated through the hydrated fat-free tissue and extra-cellular water compared with fat because of the greater electrolyte content.41 The impedance to flow of the electrical current will be directly proportional to the quantity of body fat.42 This method has not been validated for RA and is very sensitive to hydration status.43
Urinary creatinine excretion
Creatinine is the spontaneous breakdown product of creatine phosphate and creatine, which are mainly found in SM. In normal subjects, there is a strong correlation between 24-h urinary creatinine and total body SM mass.44 Ideally, the subject should consume a meat-free diet for 7 days prior to the measurement. It is, however, quick and easy to use and may be useful to detect longitudinal changes.45
Pharmacology therapeutic strategy of rheumatoid cachexia
Since the exact causes of RC are not fully delineated, no definite preventive or therapeutic strategies have been tested to-date.7 The introduction of target cytokine therapy such us anti-TNFα may provide an opportunity to address this problem, but this requires prospective assessment.46 It is a common clinical observation that RA patients receiving anti-TNFα therapy commonly and rapidly put on weight. It remains unclear whether this is due to a significant change to their body composition and reversal of RC, i.e. whether it is predominantly due to muscle gain or fat gain. Future directions may involve the co-administration of TNF α, IL-1 and anti-IL-6 blocking agents.47 In the meantime, enhanced physical activity and lifestyle changes may be a reasonable route to take in RA patients, as it is safe, associates with several other health benefits, and with concerted effort can be provided by health care systems and achieved by RA patients.48
Nutritional management of patients with rheumatoid cachexia
The conceptual focus on the pathophysiologic processes that lead to malnutrition, and not just the degree of correspondence with cut-points established for anthropometric measurements or amounts of lean mass and fat mass, is currently considered an innovative proposal for the comprehension, prevention and the management of malnutrition.49,50 With these grounds, it is possible to implement specific strategies for the management of Rheumatoid cachexia, whose main objectives are:
Physical activity prevents rheumatoid cachexia
The perceptions of people with RA may provide reasoning for the lower physical activity levels of RA patients when compared to the general population.58 Thus, understanding the perceptions of RA patients regarding exercise is salient to the role of the health professional. The Obstacles to Action study59 investigated factors influencing exercise participation for individuals with self-reported arthritis who were defined as “non exercisers”, “insufficiently active”, and “regular exercisers”. Their qualitative analysis of focus group discussions revealed that active people with arthritis believed more strongly in the benefits of physical activity, reported significantly higher levels of encouragement from others, and had greater overall levels of self-efficacy when compared with the less active participants. Arthritis, fatigue, and discomfort were ranked by both groups as the top three barriers. However, the active participants reported significantly lower impact scores for these barriers than the inactive group, and these findings persisted after adjusting for occupational status, body mass index, and comorbidities.59
Exercise programs for RA patients should be initially supervised by an experienced exercise professional so that the program can be tailored to individual aspirations and adapted to the disease activity, joint defects, and symptoms of patients.60 Following on from moderate to high intensity progressive resistance training (PRT) or combined programs, RA patients have been shown to have high adherence rates to exercise in “real life” situation that help maintain improvements.61 Although, continuation of both high-intensity and high-frequency sessions may be required for maintenance of training gains in aerobic fitness, muscle strength, and functional ability,62 but evidence is still required regarding the minimum maintenance regimen. Home-based exercise programs have also been investigated and have been shown to improve quality of life and functional status.63
However, due to the difficulties in ensuring that exercise of sufficient intensity is performed these exercises often fail to elicit significant increases in muscle strength or aerobic fitness. Although the minimal exercise dose for functional improvements and health maintenance is unknown, even regular training performed once weekly has been shown to improve function assessed subjectively by health assessment score (HAQ) scores and health status.64 As many RA patients have below average physical capacity, exercise training should be initiated at a lower intensity. Evidence of exercise prescription in RA patients with severe disability (Functional classes III and IV) is still lacking.65 Even so, strengthening exercises are recommended for all stages of RA.66 Exercise programs, even over long periods and at high intensities, have been found to be safe as well as effective.62 However, little is known as to whether exercise, particularly strength training, should be continued through inflammatory “flares” and further research should be conducted on the effects of exercise on joints that are already severely damaged. For continued training adaptation (i.e., increased fitness) a progression of the exercise dose (i.e., duration and/or intensity) is required.67
RA is a chronic inflammatory autoimmune disease with high circulating levels of cytokines and acute phase proteins. Rheumatoid cachexia is an important metabolic consequence of rheumatoid arthritis and leads to muscle weakness, disability, and loss of independence. Anti-TNF therapy and other cytokine bloquers hold promise for treating this metabolic abnormality largely ignored. However, patients with RC appeared to have adequate dietary intake in terms of calories and protein and there is no doubt, that there is a lot more evidence in favour of prescribing physical activity for patients with RA.
None.
The author declares there is no conflict of interest.

©2017 Ghozlani, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.