eISSN: 2473-0815


Research Article Volume 6 Issue 6
1Professor of Endocrinology and Diabetology Department, Siberian State Medical University, Tomsk, Russia
2PhD Student of Endocrinology and Diabetology Department, Siberian State Medical University, Russia
3Principal Researcher of Central Research Laboratory, Siberian State Medical University, Russia
4Clinical Pathologist of Tomsk Regional Perinatal Center, Russia
5Tomsk Regional Perinatal Center, Russia
6Siberian State Medical University, Russia
7Head of Laboratory Department of Tomsk Regional Perinatal Center, Russia
8Researcher Tomsk Cancer Research Institute, Russia
Correspondence: TV Saprina, MD, PhD, Professor of Endocrinology and Diabetology Department, Siberian State Medical University, Tomsk, Russia
Received: July 05, 2018 | Published: November 9, 2018
Citation: Saprina TV, Musina NN, Prokhorenko TS, et al. Pathogenetic aspects of gestational diabetes mellitus from the perspective of enteroinsulin hormones disorder: an open observational cross-sectional comparative study. Endocrinol Metab Int J. 2018;6(6):348-354. DOI: 10.15406/emij.2018.06.00210
Introduction: The frequency of carbohydrate metabolism disorders is considerably increasing all over the world. Gestational diabetes mellitus (GDM) affects between 1% and 14% of pregnant women.1 Defects in gut hormone-dependent regulation of beta-cell function (entero-insular hormonal axis) have been proposed to contribute to the pathogenesis of DM2 and gestational diabetes. However, today the state of enteroinsulin hormones (glucagon, glucose-dependent insulinotropic peptide (GIP), glucagon-like peptide 1 (GLP-1), glucagon-like peptide-2 (GLP-2) during physiological pregnancy is poorly understood, as well as their role in the development of gestational disorder of carbohydrate metabolism.
Aim: The aim of current research was to value and compare incretin hormones secretion in groups of pregnant women with normal glucose tolerance (NGT) and GDM.
Materials and methods: Research design: open observational cross-sectional comparative study. 80 patients were examined: 50 of them had GDM; the control group consisted of 30 pregnant women without GDM. Pregnant women were included in the study if they had BMI 25-40 kg/m². All patients underwent fasting glucose measurement and oral glucose tolerance test with the 75 g glucose (OGTT) in the first and second trimesters (22-24th weeks of pregnancy), as well as basal and postprandial glucagon estimation, fasting insulin and proinsulin estimation, GIP, GLP-1 and GLP-2 estimation and DNA molecular genetic testing. Insulin, proinsulin, GLP-1, GLP-2, GIP and glucagon concentrations were estimated once in that trimester, when carbohydrate metabolism disorder was diagnosed.
The main group consisted of those women who had carbohydrate metabolism disorders according to International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy as well as to Consensus on gestational diabetes mellitus in Russian Federation: diagnostics, treatment and postnatal care, 2013.
Results: Basal glucagon level in the group of women with GDM was 1503.8±528.4, in the control group – 820.3±140.1 pg/ml (p<0.05). Postprandial glucagon level in the group of patients with GDM was 527.5±76.6, in the control group – 323.3±118.6 pg/ml (p<0.05). The most considerable differences between basal and postprandial glucagon levels in comparable groups were administrated in those women who developed GDM in the first trimester of pregnancy. GLP1 level in the group of women with GDM was 2.20±1.01, whereas in the healthy group it was 0.62±0.16 ng/ml (p<0.05). An average GLP-1 level in all the
trimesters was significantly higher in women with GDM compared to the control group of patients with NGT (Table 1). The most considerable differences between GLP-1 levels in comparable groups were administrated in those patients who were diagnosed with GDM in the first and third trimesters of pregnancy. GLP2 level in the group of patients with GDM was 11.74±1.45, in the control group –10.53±0.3 ng/ml (p>0.05). The concentration of this hormone tended to be grater in those women who were included in the study in the first and second trimesters compared to those in the third trimester of pregnancy.
Conclusion:
Keywords: gestational diabetes mellitus, enteroinsulin hormones, incretins, glucose-dependent insulinotropic peptide (GIP), glucagon-like peptide 1 (GLP-1), glucagon-like peptide-2 (GLP-2), glucagon
The frequency of carbohydrate metabolism disorders is considerably increasing all over the world. Gestational diabetes mellitus (GDM) affects between 1% and 14% of pregnant women.1
Insulin resistance and β-cell dysfunction have a main role in the development of gestational diabetes mellitus (GDM). Defects in gut hormone-dependent regulation of beta-cell function (entero-insular hormonal axis) have been proposed to contribute to the pathogenesis of DM2 and gestational diabetes.
The gut incretin hormones - glucagon-like peptide-1 (GLP-1), glucagon-like peptide-2 (GLP-2) and glucose-dependent insulinotropic polypeptide (GIP) possess strong glucose-dependent insulinotropic properties and enhance glucose-induced insulin secretion from the beginning of a meal.2 This potentiation of insulin secretion after oral glucose is called the “incretin effect” and is believed to be of crucial importance for controlling postprandial glucose excursions.3 Patients with type 2 diabetes exhibit a severely reduced incretin effect4 and some studies have shown that postprandial secretion of GLP-1 may be reduced in some patients with type 2 diabetes.9,10
However, today the state of enteroinsulin hormones (glucagon, glucose-dependent insulinotropic peptide (GIP), glucagon-like peptide1 (GLP-1), GLP-2) during physiological pregnancy is poorly understood, as well as their role in the development of gestational disorder of carbohydrate metabolism. Therefore, further study of enteroinsulin hormones' role in the development of gestational carbohydrate metabolism disorder would result in a better understanding of the mechanisms of carbohydrate metabolism disorder during pregnancy and would help to find new intervention technologies of GDM risk stratification, primary prevention and therapy, based on the insulinotropic effects of incretins.
to value and compare incretin hormones secretion in groups of pregnant women with normal glucose tolerance (NGT) and GDM.
Research design: open observational cross-sectional comparative study. The study was approved by local ethics committee, registration number 3431(23.09.2013). 80 patients were examined: 50 of them had GDM; the control group consisted of 30 pregnant women without GDM. The study was held in 2014-2016 and was based in Tomsk perinatal center.
Pregnant women were included in the study if they had BMI 25-40 kg/m². All patients underwent fasting glucose measurement and oral glucose tolerance test with the 75 g glucose (OGTT) in the first and second trimesters (22-24th weeks of pregnancy). GDM was diagnosed in case of fasting plasma glucose ≥5.1 mmol/l (92 mg/dl) but <7.0 mmol/l (126 mg/dl), or postprandial plasma glucose ≥10 mmol/l in 1 hour after 75 g glucose intake, or ≥8,5 mmol/l in 2 hours after glucose intake. Those women, who were included in the study in the third trimester, underwent fasting glucose measurement and glucose estimation in 2 hours after carbohydrate-containing food intake. In case of normal fasting glucose levels and postprandial glucose levels during OGTT, patients were included in a control group. The main group consisted of those women who had carbohydrate metabolism disorders according to International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy as well as to Consensus on gestational diabetes mellitus in Russian Federation: diagnostics, treatment and postnatal care, 2013.
All women have signed an inform consent. Both groups were matched according to the age and BMI.
All patients underwent oral glucose tolerance test (OGTT), basal and postprandial glucagon estimation, fasting insulin and proinsulin estimation, GIP, GLP-1 and GLP-2 estimation and DNA molecular genetic testing. Insulin, proinsulin, GLP-1, GLP-2, GIP and glucagon concentrations were estimated once in that trimester, when carbohydrate metabolism disorder was diagnosed.
Data was analyzed using SPSS Statistics 18 software. Assuming the normal distribution of data, it was presented as mean and standard deviation (M ± SD), in other cases - as median and interquartile range (Me [Q1-Q3]). Mann-Whitney test was used for the comparison of quantitative variables; the differences were considered significant at p <0.05.
Average age in the group of women with GDM was 31.46 ±5.62 year, average age in the control group was 30.13±5.81 years. Average body mass index (BMI) in the group of women with GDM was 29.61 ±5.90 kg/m², BMI in the control group was 29.80±6.15 kg/m².
Women were included in the study in the first (12.5[11.5-13.3] weeks), second (21.0[19.0-23.0] weeks) and the third (34.3[31.0-36.7] weeks) trimesters. Incretin hormones were not estimated repetitively in the following trimesters of pregnancy.
GDM was diagnosed in the first trimester in 6 (12%) patients, in the second trimester in 26 (52%) patients and in the third trimester in 18 (36%) women. The control group consisted of 4 (13.3%) women in the first trimester of pregnancy, 21 (70%) women in the second trimester and 5 (16.7%) women in the third trimester (p>0.05).
Average fasting plasma glucose level in the group of women with GDM and in the control group was 5.4±0.5 mmol/l and 4.1±0.4 mmol/l (p<0.05) respectively. Postprandial glucose level on the 60th minute of OGTT in the main group was 7.6±1.7 mmol/l, in the control group – 6.8±1.3mmol/l (p<0.05), on the 120th minute of OGTT – 6.3±1.3 and 5.9±1.2 mmol/l respectively (p<0.05).
Mean levels of incretin hormones, insulin, proinsulin and glucagon among women of both groups are showed in the Table 1.
|
GDM |
Control group |
p |
HbA1c,% |
4.5±0.6 |
4.5±0.5 |
>0.05 |
Fasting insulin, mU/L |
12.2±1.8 |
12.1±1.6 |
>0.05 |
Fasting proinsulin, mU/L |
3.8±0.9 |
2.5±1.6 |
0.05 |
Basal glucagon, pg/ml |
1503.8±528.4 |
820.3±140.1 |
0.01 |
Postprandial glucagon on the 30th minute of OGTT, pg/ml |
527.5±76.6 |
323.3±118.6 |
>0.05 |
Fasting GLP-1, ng/ml |
2.2±1.0 |
0.6±0.2 |
0.002 |
Fasting GLP-2, ng/ml |
11.74±1.5 |
10.5±0.3 |
>0.05 |
Fasting GIP, ng/ml |
29.9±3.2 |
33.6±3.6 |
>0.05 |
Table 1 HbA1c level and enteroinsulin hormones` (glucagon, GIP, GLP-1, GLP-2) concentrations among women with GDM and control group patients (M±SD)
Basal glucagon level was significantly higher in the group of women with GDM, than in the control group (Table 1). Postprandial glucagon level, administrated on the 30th minute of OGTT was higher in the GDM group, than in women with normal glucose tolerance (NGT), though the difference was not significant.
The most considerable differences between basal and postprandial glucagon levels in comparable groups were administrated in those women who developed GDM in the first trimester of pregnancy. Basal glucagon level in the first trimester among NGT women (control group) was 698.55[401.8 –874.45] pg/ml, in the main group–3638.4[343.8-3834.60] pg/ml; postprandial glucagon level in these groups was 389.3[291.5-402.11] pg/ml and 379.068[20.360-612.14] pg/ml respectively.
There was no significant difference in basal and postprandial glucagon levels in those patients who developed GDM in the second and third trimesters (Figure 3) (Figure 4).
No significant difference was found between fasting insulin levels in the GDM group and NGT group. Proinsulin level was higher in women with GDM (p=0.05).
As it was already mentioned, women in both groups were overweight or had class I-II obesity.
Figures 1-9 and Table 2 represent the dynamic of fasting glucose, glycated hemoglobin (HbA1c), incretin hormones (GIP, GLP-1 and GLP-2) concentrations, insulin/proinsulin, basal and postprandial glucagon concentrations among patients of the main and the control group.
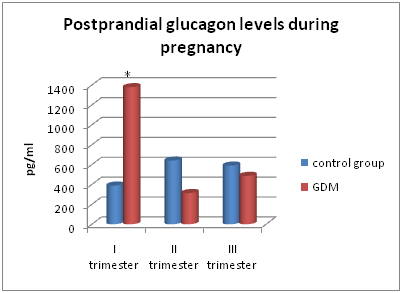
Figure 6 Concentrations of postprandial glucagon on the 30th minute of OGTT in the main and control groups.
|
1 trimester |
2 trimester |
3 trimester |
|||
|---|---|---|---|---|---|---|
GDM |
Control group |
GDM |
Control group |
GDM |
Control group |
|
Fasting glucose, mmol/l |
4.7±0.7 |
4.6±0.3 |
4.3±0.6* |
3.9±0.4 |
4.9±1.2 |
4.1±0.3 |
HbA1c,% |
4.5±0.6 |
4.4±0.3 |
4.5±0.5 |
4.4±0.3 |
4.6±0.36* |
5.1±1.1 |
Fasting insulin, mU/L |
7.7±2.6 |
12.4±7.2 |
9.9±8.9 |
12.5±9.7 |
17.2±7.9 |
9.9±6.7 |
Fasting proinsulin, mU/L |
2.5±0.7 |
2.9±1.3 |
4.8±3.3 |
2.6±1.8 |
2.9±1.6 |
2.1±0.4 |
Basal glucagon, pg/ml |
3638.4±866.2* |
698.6±223.8 |
968.3±134.6 |
995.3±451.8 |
720.5±604.4 |
748.7±407.1 |
Postprandial glucagon on the 30th minute of OGTT, pg/ml |
1379.1±177.1* |
389.3±66.2 |
313.7±59.9 |
641.1±221.5 |
487.1±68.7 |
590.4±94.8 |
Fasting GLP-1, ng/ml |
1.12±0.73* |
0.03±0.01 |
0.67±0.61 |
0.37±0.29 |
1.01±0.17* |
0.52±0.24 |
Fasting GLP-2, ng/ml |
10.6±1.8 |
10.1±0.6 |
10.3±2.6 |
9.8±3.1 |
9.4±1.1 |
9.6±1.3 |
Fasting GIP, ng/ml |
33.6±5.6 |
32.8±1.4 |
32.2±5.4 |
34.2±3.9 |
31.1±6.3* |
37.3±5.6 |
Table 2 In the table and in the figure 3-7 * -p<0.05 compared to the control group.
Fasting glucose levels and HbA1c levels during pregnancy are represented in Figure 1 & Figure 2. Though there was no significant difference in insulin and proinsulin levels between studied groups, we found out an increase of insulin concentration from the first to third trimester of pregnancy and higher proinsulin level among those women who developed GDM in the second trimester. At the same time, women with NGT had higher insulin and proinsulin levels in the first and second trimesters with a decrease of insulin concentration from the first to third trimester of pregnancy (Figure 3) (Figure 4).
Figures 5-6 demonstrate glucagon levels on 0th and 30th minutes of OGTT in women of both groups. Those women who developed GDM in the first trimester of pregnancy had significantly greater basal and postprandial glucagon levels, as well as fasting GLP-1 level (p<0.05), compared with control women (Figure 7).
An average GLP-1 level in all the trimesters was significantly higher in women with GDM compared to the control group of patients with NGT (Table 1).
The most considerable differences between GLP-1 levels in comparable groups were administrated in those patients who were diagnosed with GDM in the first and third trimesters of pregnancy. In the group of women with normal glucose tolerance fasting GLP-1 level tended to be higher among patients in the second and third trimesters being maximal among women in the third trimester (Figure 7).
No significant difference was observed in GLP-2 levels between patients with NGT and those with GDM. The concentration of this hormone tended to be grater in those women who were included in the study in the first and second trimesters compared to those in the third trimester of pregnancy (Figure 8).
The fasting secretion of GIP hormone in the group of patients with NGT was higher among women examined in the third trimester of pregnancy compared to those in the second and first trimesters. The situation was different in the main group. In GDM patients the secretion of GIP was maximal among women in the first trimester of pregnancy and minimal among women in the third trimester. The difference in fasting GIP secretion between the main and the control groups was significant for women included in the study in the third trimester of pregnancy (Figure 9).
Gestational diabetes mellitus is considered to develop if pancreatic beta cells are unable to face the increased insulin demand in the second and third trimesters of pregnancy, when gluconeogenesis is greater due to placental factors. Thus, GDM is more likely to occur in the second and the third trimesters, than in the first one. The development of carbohydrate metabolism disorders in the first trimester of pregnancy is discussed as MODY type of the disease or autoimmune diabetes manifestation. In our research we investigated one of possible mechanisms of gestational glucose intolerance – enteroinsulin hormones disorder, as well as higher basal and postprandial glucagon secretion.
In our research those women, who developed GDM in the first trimester of pregnancy according to the new diagnostic criteria (fasting glucose level higher than 5,1 mmol/L) had grater basal and postprandial glucagon levels.
A recent report (Grigorakis SI, 2000) showed that women with GDM had higher glucagon levels compared to non-diabetic group.5 A research held in 2005 (Beis C) demonstrated the same results: basal glucagonemia was grater in GDM-group of patients, than in the control group (healthy pregnant women, matched to the main group by age and BMI).6
Those patients, who developed GDM in the first trimester and had fasting and postprandial hyperglucagonemia, also tended to have significantly greater basal GLP-1 concentrations.
It was proposed that elevated GLP-1 secretion during OGTT in the i-IFG (isolated impaired fasting glycaemia) individuals represents a compensatory mechanism to maintain near normal absolute insulin secretion rates at the earliest stages of abnormal glucose homeostasis, but that this compensation may be lost again when (postprandial) glucose levels increases.7
Our data clearly show that women with GDM had higher fasting GLP-1 level compared to NGT-patients, and this is consistent with the findings of previous studies (Cypryk K).7,8
However, these results seem to be in contrast with other researches: a reduced GLP-1 secretion was observed among patients with GDM compared to healthy pregnant women. The same tendency remained postpartum.9 A research, held in 2013, established that pregnancy itself is associated with reduced postprandial GLP-1 responses compared to GLP-1 response postpartum.10
It is known that proglucagon gene expression in the intestinal L cells generates proglucagon (PG) that is processed by prohormone convertases (PC1/3) to liberate the GLP-1(1–37) peptide.11,12 Although glucagon gene expression also generates PG in islet α-cells, it was thought that α-cells fail to synthesize GLP-1 due to the fact that these endocrine cells contain a prohormone convertase (PC2) that preferentially processes PG to glucagon.13 However, it is now apparent that endocrine cell “plasticity” exists within the islets such that α-cells synthesize GLP-1 under stressful or pathophysiological conditions including T2DM.14,15 This fact can explain increased GLP-1 concentrations among women with GDM that were established in several studies. On the other hand, greater GLP-1 levels among women with impaired carbohydrate metabolism may reflect an incretin resistance similar to compensatory hyperinsulinemia associated with insulin resistance in patients with T2DM manifestation.
The research of Shestakova, held in 2014, demonstrated higher glucagon levels among patients with impaired fasting glucose (IFG). These results show a greater impact of glucagon on fasting glucose concentration, compared to postprandial one.16
The available data characterizing the dynamic of glucagon secretion during physiological pregnancy is rather ambiguous. However, it was showed that baseline and 3 h after glucose ingestion, plasma glucagon levels were significantly higher in women with GDM compared to normal pregnant women.6 Interestingly, the same investigation demonstrated a significant increase of postglucose plasma glucagon levels at 1 and 2 h compared to baseline levels in normal pregnancy, while there was no change in GDM pregnancy.
Lack of suppression of plasma glucagon levels among DM patients may indicate the glucagonotropic effect of GLP-2. Thus, it was demonstrated that the intravenous infusion of GLP-2 led to a marked increase in glucagon concentrations both in the fasting state and during the meal study, counteracting the glucagonostatic effect of GLP-1.17,18,19,20
In our research women, who were diagnosed GDM in the first trimester, tended to have greater basal GLP-2 levels, though the data were not significant. However, fasting GLP-2 concentrations were higher in GDM patients compared to overweight and obese NGT women. This may suggest that GLP-2 has a significant impact on the regulatory mechanisms of carbohydrate metabolism. Due to its glucagonotropic effect, GLP-2 may be considered as one of anti-incretin hormones.
Those women, who were diagnosed GDM in the second trimester, had increased fasting proinsulin secretion compared to non-diabetic patients, though the data were not significant.
Higher basal GLP-1 and insulin concentrations, as well as decreased GIP secretion, were observed in a group of women, who developed GDM in the third trimester of pregnancy compared to NGT group.
GIP is considered to be a bifunctional glucose-dependent regulator of glucagon and insulin secretion. Thus, GIP exhibits glucagonotropic effects during fasting and hypoglycemic conditions when little or no effect on insulin secretion is exerted by the hormone. In contrast, GIP has no effect on glucagon secretion during hyperglycemia, when it robustly potentiates glucose-induced insulin secretion.21,22,23 In our research the women, who developed GDM in the third trimester, had higher insulin secretion compared to non-diabetic group, while there was no significant difference between basal and postprandial glucagon levels. These results indicate the prevalence of insulinotropic effects of GIP in impaired carbohydrate metabolism conditions.
Consequently, a further study of enteroinsulin hormones' role in the development of gestational carbohydrate metabolism disorder would result in a better understanding of the mechanisms of carbohydrate metabolism disorder during pregnancy and would help to find new intervention technologies of GDM risk stratification, primary prevention and therapy, based on the insulinotropic effects of incretins.
The research is financed withgrantRussian Fund of Fundamental Investigations (RFFI) р_а № 16-44-700246.
None.
The author declares there is no conflict of interest.

©2018 Saprina, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.