eISSN: 2473-0815


Case Report Volume 2 Issue 4
Department of Clinical Medicine, Health Center of Cape Huertas, Spain
Correspondence: Salvador Pertusa, Department of Clinical Medicine, Health Center of Cape Huertas, Alicante, Spain
Received: October 21, 2015 | Published: November 25, 2015
Citation: Pertusa S, Montserrat P, David M, et al. MODY 2 diabetes: an unusual presentation of diabetes. Endocrinol Metab Int J. 2015;2(4):166-169. DOI: 10.15406/emij.2015.02.00032
Diabetes mellitus includes a heterogeneous mix of metabolic diseases characterized by chronic hyperglycemia. The most common varieties of diabetes mellitus are Type 1 and Type 2, but there are other types. MODY diabetes (Maturity Onset Diabetes of the Young) is a clinically heterogeneous disease characterized by non-dependent insulin diabetes diagnosed early with dominant autosomal transmission and absence of auto-antibodies. It is the most frequent form of monogenic diabetes. Many patients are misclassified as Type 1 or Type 2 diabetes. In this paper, we are describing the case of a 51 year old male patient diagnosed with MODY 2 diabetes. The primary care physician should be aware of the existence of other types of Diabetes Mellitus different from the more common diabetes type 1 and type 2, and the importance of other cardiovascular risk factors such as hypercholesterolemia, hypertension or smoking.
Keywords: MODY, maturity onset diabetes of the young, diabetes mellitus, hyperglycemia, polymorphism, type 1 or type 2 diabetes, metabolic disease, glycaemia, cardiac arrest, genes, glicazide, medical therapy, encephalic hypoxia sequelae, pathalogical characteristics, insulin
MODY, maturity onset diabetes of the young; DM, diabetes mellitus; ICA, islet-cell antibodies; GAD, glutamic acid decarboxylase; IA-2, insulinoma-associated protein 2
Diabetes mellitus includes a heterogeneous mix of metabolic diseases characterized by chronic hyperglycemia. Some forms of this illness respond to specific etiological or pathogenic characteristics, but the underlying etiology is unknown. The most common varieties of diabetes mellitus are Type 1 and Type 2, but there are other types. Numerous common polymorphisms (approximately 50 to date) weakly contribute to the risk for or protection from type 2 diabetes. The genes encode proteins that cause alterations in several pathways leading to diabetes, including pancreatic development, insulin synthesis, processing, and secretion; amyloid deposition in beta cells; cellular insulin resistance; and impaired regulation of gluconeogenesis. In this article, we are focusing on the functional congenital defects of beta cells usually present in these not so frequent types of diabetes.
MODY diabetes (Maturity Onset Diabetes of the Young) is a clinically heterogeneous disease characterized by non-dependent insulin diabetes diagnosed early (< 25 years of age) with dominant autosomal transmission and absence of auto-antibodies.1 It is the most frequent form of monogenic diabetes, representing 2% –5% of diabetes cases.2,3 It is estimated that this form represents 68 to 108 cases per million people.4 Patients present a heterogeneous population making extremely difficult to predict an underlying pathogenesis.5,6 Many patients are misclassified as Type 1 or Type 2 diabetes. Several different genetic abnormalities have been identified, each leading to a different type of disease (Table 1). The subtypes of MODY are defined by specific descriptions of the known genetic defects. The genes involved control pancreatic beta cell development, function, and regulation, and the mutations in these genes cause impaired glucose sensing and insulin secretion with minimal or no defect in insulin action7-9 In this paper, we are describing the case of a 51 year old male patient diagnosed with MODY 2 diabetes.
Type |
Genetic Defect |
Frequency |
Beta Cell Defect |
Clinical Features |
Risk of Microvascular Disease |
Optimal Treatment |
1 |
Hepatocyte nuclear factor-4-alpha |
<10% |
Reduced insulin secretory response to glucose |
Normal renal threshold for glucose |
Yes |
Sulfonylureas |
2 |
Glucokinase gene |
15% - 31% |
Defective glucokinase molecule (glucose sensor), increased plasma levels of glucose are necessary to elicit normal levels of insulin secretion |
Mild, stable, fasting hyperglycemia, often diagnosed during routine screening. Not progressive. |
Generally no |
Diet |
3 |
Hepatocyte nuclear factor-1-alpha |
52% - 65% |
Abnormal insulin secretion, low renal threshold for glucose |
Low renal threshold for glucose+glycosuria |
Yes |
Sulfonylureas |
4 |
Insulin promoter factor 1 |
Rare |
Reduced binding to the insulin gene promoter, reduced activation of insulin gene in response to hyperglycemia |
Rare, pancreatic agenesis in homozygotes, less severe mutations result in mild diabetes |
Yes |
|
5 |
Hepatocyte nuclear factor-1-beta |
Rare |
|
Pancreatic atropy, renal dysplasia, renal cysts, renal insufficiency, hypomagnesemia |
Yes |
Insulin |
6 |
Neurogenic differentiation factor-1 |
Rare |
Pancreatic development |
|
Yes |
Insulin |
Table 1 Maturity onset diabetes of the young: More commonly identified gene mutations
A fifty one year old male presented for a routine blood test. The test results showed: Glucose: 178mg/dl, Cholesterol: 307mg/dl (100-200), LDL-cholesterol: 218mg/dl (0-155) and HbA1c 6.5%. Family history: mother with Type 2 Diabetes mellitus, and dyslipidemia and father with myocardial infarction at 72 years old. Statin treatment was started and a new confirming glycaemia blood test was requested. Two weeks later: Glucose: 136mg/dl, and HbA1c: 6.6%. Due to the initial diagnosis of Type 2 Diabetes mellitus, he started treatment with Metformin, Atorvastatin, and changes in hygiene and diet were implemented. In addition, a 6 months follow up blood test control was scheduled.
The day after diagnosis the patient fell suddenly to the ground while exercising, resulting in loss of consciousness and cardiac arrest. His friends performed 20 minute cardiopulmonary resuscitation on him and subsequently he was assisted by the emergency staff. After detecting ventricular fibrillation, they applied advanced resuscitation treatment. Patient arrived at the hospital with severe signs of hypoxia. Urgent coronary catheterization was performed due to an extensive myocardial infarction, and coronary stents were placed.
During clinical follow up, patient commented that in the previous 17 years he had glycemic levels of around 137mg/dl. Under his own initiative and because his brother was a young doctor and prescribed him this medication, he started a diet and a medical therapy with Glicazide when he was 34 years old without further medical follow up controls. The outcome was favorable despite Encephalic Hypoxia sequelae, loss of a third of the lower visual field, and loss of memory. Subsequently the patient has also presented several angina episodes. A thorough endocrinological study found monogenic diabetes due to a pGlu265Lys mutation on the glucokinase gene and no other disorders;
Monogenic causes of type 2 diabetes mellitus represent a small percentage of cases. Usually, inherited polymorphisms contribute in an individual basis, with a low risk to cause or prevent onset of diabetes. Type 2 diabetes’ genetic risk is due, in most cases, to complex polygenic risk factors.
Various genetic anomalies have been identified, each one causing a different type of the illness. Mutations in the factor 1 alpha gene hepatocyte core (HNF-1A) and the glucokinase gene are the ones frequently identified, appearing in 52-65% and 15-32% of MODY diabetes cases, respectively.10,11
More than a dozen glucokinase gene mutations of chromosome 7 have been described.12 Defects on the expression of this enzyme (Figure 1), which incorporates phosphor to glucose resulting in 6 glucose-phosphate (which probably serves as a glucose sensor,) causes an increase of the threshold for the onset of insulin secretion stimulated by glucose. Sometimes, the expressed enzyme works but is unstable, which leads back to a deficit in insulin secretion.13 The resulting hyperglycemia is often stable, and is not associated with vascular complications, which are so frequent in other types of Diabetes mellitus.14 Patients with this glucokinase gene mutation can be usually controlled by diet. If necessary, the medical treatment of choice is with sulfonylureas.
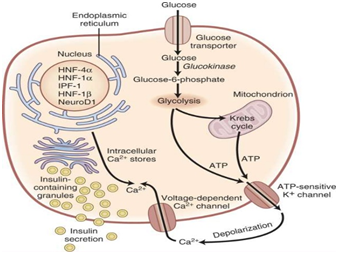
The diagnosis of MODY diabetes is performed by genetic tests that determine the direct sequencing of the gene. It is important to differentiate MODY Diabetes from Types 1 and 2 Diabetes (Table 2) because the optimal treatment and the risk of diabetic complications change based on the underlying genetic defect. As an example, patients with MODY due to HNF1A or HNF4A mutations are frequently misdiagnosed as having insulin requiring type 1 diabetes because they present at an early age and are not obese. However, many of these patients can be successfully managed with sulfonylureas monotherapy. In addition, distinguishing MODY from type 1 and type 2 diabetes allows earlier identification of at risk family members. It would be interesting to perform genetic testing for MODY when there is a high index of suspicion (familial diabetes with autosomal dominant pattern of inheritance (>2 generations), onset <25 years, non-obese, negative islet autoantibodies).
In all patients, therefore, it is important to obtain a detailed history of diabetes at diagnosis, including age, body mass index (BMI), and presenting symptoms.15 It is also important to ascertain insulin dependency and the presence or absence of family history of diabetes.
In a patient with presumed type 1 diabetes, measurement of serum autoantibodies (islet-cell antibodies [ICA], glutamic acid decarboxylase [GAD] 65, insulin, tyrosine phosphatases, insulinoma-associated protein 2 (IA-2) and IA-2 beta) should be performed prior to consideration of genetic testing for MODY. The presence of autoantibodies makes MODY very unlikely.16
It is more difficult to differentiate between MODY and type 2 diabetes mellitus. For patients with presumed type 2 diabetes, the presence of a simple (non-multigenerational) family history does not discriminate between MODY and type 2 diabetes. Insulin resistance is not a feature of MODY. Thus, diabetes in the absence of obesity is suspicious for MODY, particularly in adolescents with presumed type 2 diabetes. However, the absence of obesity or surrogate markers of insulin resistance is, in general, a poor discriminator of MODY and type 2 diabetes in adults.17 There are currently no biochemical tests that reliably differentiate between the two diseases.
Clinical Characteristics |
Type 1 Diabetes |
Type 2 Diabetes |
MODY 2 Diabetes |
Age at time of diagnosis (years) |
Most cases < 25, despite it could take place at any age |
Typically > 25, but incidence is increasing among teenagers as obesity rates increase in children and adolescents |
< 25 |
Weight |
Usually slender but overweight and obese cases on the rise at time of diagnosis |
>90% of cases are overweight |
Similar to general population |
Auto-Antibodies |
Present |
Not present |
Not present |
Insulin dependence |
Yes |
No |
No |
Insulin sensitivity |
Normal if under control |
Diminished |
Normal (diminished when obesity is present) |
Diabetes in family members |
Not frequent (5-10%) |
Frequent (75-90%) |
Multigenerational, >2 generations |
Risk of diabetic Ketoacidosis |
High |
Low |
Low |
Table 2 Clinical characteristics that are differentiators between Types 1 and 2 Diabetes and MODY 2 (Mature age diabetes present during youth) Diabetes
For family members of mutation carriers, biochemical testing to confirm diabetes should be performed before genetic testing is considered.18 If the biochemical tests are consistent with a diagnosis of diabetes, genetic testing can be performed to confirm the diagnosis of a MODY mutation. Two of the children of this patient were mutation carriers though did not have the disease.
One wonders whether Diabetes mellitus in this patient has been an important contributor to his vascular complications. Based on the reasons exposed above, and despite what we thought initially, the most likely answer would be no. We believe that his sustained high levels of hypercholesterolemia for a long period of time would have had greater weight in his complications.
As for the current antidiabetic treatment received by the patient, we believe it is not the most adequate. The patient began treatment with Metformin based on the initial Type 2 Diabetes mellitus diagnosis. This treatment has continued despite the new diagnosis of MODY Diabetes. The decrease of the hepatic glucose production, which is the mechanism of action of this drug, would be unsatisfactory for the new diagnosis. On the contrary, we think that Sitagliptin could play a controlling role against this patient’s diabetes due to its stimulating effect on endogenous insulin secretion by inhibiting the dipeptidilpeptidase 4 enzyme but currently there is no evidence in that way yet. A differential diagnosis was established with a transitory ischemic attack and after the corresponding assessment was ruled out.
Finally, one may wonder whether it is necessary screening of monogenic diabetes in primary care. Although not very common, it would be interesting to keep in mind this type of Diabetes Mellitus in Primary Care. Diabetes in the absence of obesity is suspected of MODY diabetes, especially in adolescents where it is thought to have type 2 diabetes.
This is a case of MODY type of Diabetes mellitus that presented initial diagnosis difficulties. The primary care physician should be aware of the existence of other types of diabetes mellitus different from the more common diabetes type 1 and type 2, and the importance of other cardiovascular risk factors such as hypercholesterolemia, hypertension or smoking. In this case, we intend to sensitize family doctors to start actively looking for other types of diabetes mellitus if the clinical situation that presents itself is unclear.
None.
The author declares that there are no conflicts of interest.

©2015 Pertusa, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.