eISSN: 2473-0815


Case Report Volume 5 Issue 4
1Department of Medicine, University of Miami Miller School of Medicine, USA
2Department of Neurology and Neurosurgery, University of Miami Miller School of Medicine, USA
Correspondence: Atil Y Kargi, Division of Endocrinology, Diabetes and Metabolism, Department of Medicine, University of Miami Miller School of Medicine, Miami, FL 33136, USA
Received: July 23, 2017 | Published: October 4, 2017
Citation: Hannoush ZC, Palacios JD, Yavagal D, et al. IPSS in cyclical cushing’s syndrome due to ectopic ACTH production: proof of principle and potential diagnostic pitfall. Endocrinol Metab Int J. 2017;5(4):271-273. DOI: 10.15406/emij.2017.05.00131
Introduction: Cyclical Cushing’s syndrome (CCS) has been described in patients with excessive endogenous cortisol produced in fluctuating rhythmical patterns. Although most cases of CCS have been reported secondary to an adrenocorticotropic hormone (ACTH) producing pituitary adenoma, there have also been reports of CCS caused by ectopic ACTH producing tumors. Due to its fluctuating nature, the clinical and biochemical diagnosis of CCS represents a challenge.
Case presentation: A 53 year-old man undergoing evaluation for ACTH-dependent CCS underwent inferior petrosal sinus sampling (IPSS). The peak inferior petrosal to peripheral ACTH gradient level was consistent with a false-positive diagnosis for Cushing’s disease (CD). However, results showed suppression of the hypothalamic-pituitary-adrenal (HPA) axis from antecedent hypercortisolism, therefore the IPSS was deemed non-diagnostic. Repeat IPSS during another cycle of hypercortisolism a few months later, confirmed the diagnosis of ectopic ACTH production. The source was localized to the lung and the patient underwent resection of a typical bronchial carcinoid tumor staining for ACTH. The patient achieved complete biochemical and clinical resolution of Cushing’s syndrome (CS).
Discussion and conclusion: While the potential for IPSS to result in diagnostic misclassification of the etiology of CCS has been brought up in the literature, to the best of our knowledge, this is the first report of IPSS performed both in and out of cycle in a patient with CCS due to ectopic ACTH syndrome. The findings could provide “proof-of-principle” as to the expected findings and the potential for false-positive results leading to erroneous diagnosis of pituitary etiology of CCS.
Keywords: cyclical cushing’s syndrome, cushing’s syndrome, inferior petrosal sinus sampling (IPSS), diagnosis, ectopic ACTH
Cyclical Cushing’s syndrome (CCS) has been described in patients with Cushing’s syndrome in which excessive endogenous cortisol is produced in fluctuating rhythmical patterns1{Mullan, 2007 #16}. This syndrome can be associated with fluctuating signs and symptoms; cycle lengths have been described between 12 hours and 85 days.2 CCS was initially believed to be very rare, but recent evidence has shown that it might occur more frequently that predicted.3 Although most cases of CCS have been reported secondary to an adrenocorticotropic hormone (ACTH) producing pituitary adenoma, there have also been reports of adrenal adenomas, ectopic typical and atypical carcinoids, pheochromocytomas and other ectopic ACTH producing tumors.2 The pathophysiologic mechanisms behind the fluctuations in hormone production are not well understood although different mechanisms have been proposed based on findings from case reports.1
The clinical and biochemical diagnosis of CCS can represent a challenge for the clinician. Here we report the case of a 53 year-old man with cyclical Cushing’s syndrome due to an ectopic ACTH producing typical bronchial carcinoid tumor and discuss the challenges in its diagnosis, focusing on the potential diagnostic pitfall in the interpretation of the inferior petrosal sinus sampling (IPSS) which if performed “out-of-cycle” can lead to misleading conclusions regarding the source of excess ACTH production.
A 53 year-old man with history of recent onset hypertension and type 2 diabetes mellitus, presented with progressively worsening symptoms of depression, lack of energy, muscle weakness and hypokalemia. He was admitted to an outside hospital with altered mental status due to severe hypokalemia and hyperammonemia with normal liver function. Laboratory workup revealed significant hypercortisolism: 24h urine free cortisol (UFC) >4000 mcg/24h (n < 55 mcg/24h), AM cortisol of 60 mcg/dL (n 4-22 mcg/dL), PM cortisol of 30 mcg/dL, elevated ACTH level of 257 pg/mL (n 6-50 pg/mL) with failure to suppress cortisol after low and high dose dexamethasone suppression testing, normal DHEA-S and thyroid function tests. Whole body CT scan and octreotide scan as well as pituitary MRI failed to reveal any tumors, the adrenal glands where found to have bilateral hypertrophy without discrete lesions. The patient had no history of receiving exogenous steroids, denied use of valproic acid, ethanol, or other substances. After his discharge the patient improved significantly going back to his clinical baseline. At that time in the outpatient setting he had normal laboratory values with a 24 h UFC of 21 mcg/24 h, AM serum cortisol of 17.4 mcg/dL, ACTH of 46 pg/mL, DHEA 104 ug/dL, aldosterone of 10 ng/dL and plasma renin activity of 3.27 ng/mL/hr while on a beta blocker and normal electrolytes. Pertinent findings on physical exam included no moon facies but slight plethora, some supraclavicular fullness, no dorsocervical fat pad, obese abdomen with no organomegaly and no striae.
Follow up laboratory workup revealed significantly elevated levels of cortisol and ACTH alternating with normal values. The patient was observed to have symptoms of irritability, mental cloudiness, plethora and worsening hypertension during the episodes of hypercortisolism. Cycles of hypercortisolism would occur approximately every 4 months and last almost 4 weeks. The patient was scheduled for IPSS. 2 weeks prior to IPSS: UFC >2400 mcg/24h, plasma ACTH concentration of 190 pg/mL and 8 A.M. serum cortisol 35.9 mcg/dL. Hypercortisolemia was re-confirmed 5 days prior to IPSS.
During IPSS baseline ACTH concentrations were similar at femoral vein (14 pg/mL), right IPS (11 pg/mL) and left IPS (17 pg/mL) (Table 1). Following corticotropin-releasing hormone (CRH) stimulation, ACTH levels peaked at 41 pg/mL in the right IPS and 30 pg/mL in the left IPS while there was no increase in ACTH at the femoral vein. The peak IPS:P ratios were 4.1 on the right and 2.4 on the left. Cortisol levels remained low (< 4.2 mcg/dL) after CRH stimulation. Though peak IPS:P ACTH gradient was >3, commonly accepted as indicative of Cushing’s disease (CD), the results were interpreted as consistent with suppression of the HPA axis from antecedent hypercortisolism, therefore the IPSS was deemed as non-diagnostic for subtype classification of ACTH-dependent CS.
Site |
Femoral vein |
Right IPS |
Left IPS |
Right IPS:P |
Left IPS:P |
|
Minutes after CRH |
ACTH |
Cortisol |
ACTH |
ACTH |
ACTH |
ACTH |
0’ |
14 |
11 |
17 |
0.8 |
1.2 |
|
2’ |
8 |
3.3 |
13 |
10 |
1.6 |
1.3 |
5’ |
11 |
12 |
1.1 |
|||
10‘ |
11 |
3.6 |
36 |
14 |
3.3 |
1.3 |
15’ |
10 |
3.5 |
41 |
24 |
4.1 |
2.4 |
30’ |
13 |
4.1 |
19 |
30 |
1.5 |
2.3 |
Table 1 Results of first IPSS performed “out-of-cycle” with low ACTH and cortisol measurements at all sites sampled and all time points with increase of ACTH in the petrosal sinuses after CRH stimulation yielding a peak IPS: P ACTH gradient >4. Units for serum concentrations: ACTHng/mL, Cortisolmcg/dL
The patient presented with another cycle of severe hypercortisolism over 4 months after the initial IPSS. Repeat IPSS showed elevated baseline ACTH (99 pg/mL) and cortisol (45 mcg/dL) levels at the femoral vein (Table 2). Due to thrombotic occlusion, the left IPS was unable to be accessed during the second IPSS procedure. All IPS:P ratios were < 1.4 before and after CRH administration and ACTH and cortisol levels did not rise at any site.
Site |
Femoral Vein |
Right IPS |
Right IPS:P |
|
Minutes after CRH |
ACTH |
Cortisol |
ACTH |
|
0’ |
99 |
44 |
80 |
0.8 |
2’ |
82 |
43 |
||
5’ |
79 |
84 |
1.1 |
|
10‘ |
69 |
45 |
63 |
0.9 |
15’ |
70 |
44 |
51 |
0.7 |
30’ |
73 |
39 |
78 |
1.1 |
Table 2 Results of second IPSS performed “in cycle” demonstrating elevated ACTH (reference range 6-55pg/mL) and hypercortisolemia. No significant IPS: P ACTH gradient was observed before or after CRH administration and there was no significant increase in ACTH or cortisol following CRH stimulation. Results were consistent with an ectopic source of ACTH production. The left inferior petrosal sinus was not accessible due to occlusion from thrombus. Units for serum concentrations: ACTHng/mL, Cortisolmcg/dL
The patient was diagnosed as ectopic ACTH syndrome (EAS). Further imaging with repeat octreoscan and FDG-PET/CT were negative, the patient was subsequently referred to National Institutes of Health in Bethesda, Maryland for further imaging investigation which included lung MRI, F-DOPA PET and Gallium dotatate PET imaging studies which revealed a 1 cm right middle lobe pulmonary lesion and the patient underwent right middle lobe wedge lung nodule lobectomy revealing a 1.3 cm typical bronchial carcinoid tumor staining for ACTH (Figure 1). Postoperatively, he had low cortisol levels and was started on physiologic replacement dose of hydrocortisone that in a period of 3 months was safely tapered until discontinued. The patient showed complete resolution of his signs and symptoms of Cushing's syndrome and continues to be on biochemical remission after 2 years.
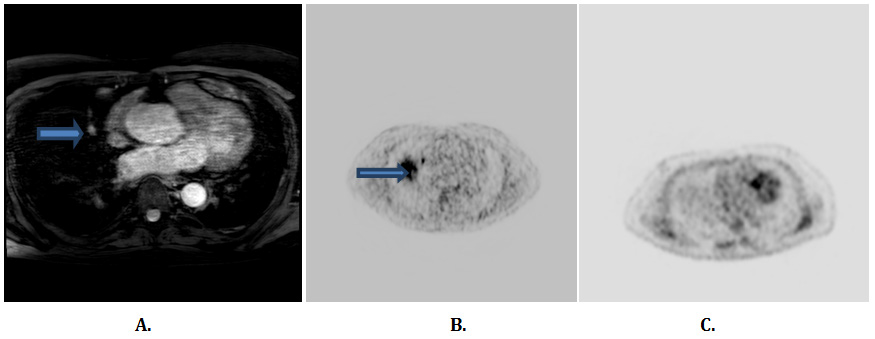
Figure 1 Results of imaging studies for the investigation of ectopic ACTH production. (A) Lung MRI demonstrates 1 cm right middle lobe lung nodule; (B) F-DOPA PET image demonstrating increased radiotracer uptake in the location consistent with the lung nodule on MRI; (C) FDG-PET scan does not show a focus of increased activity in the lungs
Cyclical Cushing’s syndrome often poses a diagnostic challenge. It should be suspected in patients with typical features of Cushing’s syndrome with normal or variable biochemical findings of ACTH and cortisol level, in cases of clear signs and symptoms of CS and evidence of adrenal insufficiency and patients with biochemical evidence of hypercortisolism without overwhelming clinical features of CS. Exogenous cortisol intake, pseudo-Cushing’s states, glucocorticoid resistance and macronodular adrenal hyperplasia should be ruled out in these situations as part of the differential diagnosis.4
CCS has been defined as a biochemical finding of at least three peaks and two troughs of cortisol production,5 but variabilities in the inter-cyclic period brings a substantial limitation to the diagnosis. Not all patients with CCS have consistent cycle length and we would propose a change in nomenclature for such cases from CCS to the more accurate term “episodic hypercortisolism”. High-dose dexamethasone suppression test can be misleading and there have been cases reported with paradoxical response.2 Frequent repeat collection of 24 h urine for free cortisol measurement is inconvenient for many patients. Different diagnostic approaches have been proposed including measurement of cortisol to creatinine ratio of morning urine samples for 28 consecutive days, late night salivary cortisol measurement during the same time period,6 as well as the use of the desmopressin test,4 and new procedures that have now become available such as the measurement of hair cortisol levels.7 However multiple concerns remain about the precision and accurate interpretation of these tests.
Once the diagnosis of ACTH dependent Cushing’s syndrome has been established, bilateral inferior petrosal sinus sampling can be performed to try differentiating central (pituitary) from ectopic sources of ACTH production8. However, this procedure can be unreliable in CCS unless performed when the syndrome is active. It is recommended that a morning serum and a late-evening salivary cortisol or 24 hour UFC be measured and shown to be increased prior to proceeding with IPSS. Prior authors have suggested that out-of-cycle IPSS in cyclical disease of other causes than pituitary could cause a false-positive for Cushing disease due to lack of suppression of corticotrophs, meaning that since the corticotrophs are no longer suppressed by high cortisol, after giving CRH the ACTH and cortisol would increase.2 In a review of the value of prolactin measurement in IPSS to document adequate catheterization of the petrosal sinuses, Sharma and colleagues provided a brief description of potentially false-positive IPSS results for CD in a patient later diagnosed as EAS.9 The ipsilateral IPS:P prolactin ratio was reported as low suggesting possible inadequate catheterization of the IPS and a repeat IPSS during a cycle of hypercortisolism was not necessary as the patient was subsequently diagnosed by other means as EAS. While a limitation of our report is that prolactin levels were not measured during IPSS and the left IPS was unable to be accessed due to occlusion during the “in-cycle” IPSS study, we were able to demonstrate results of IPSS when performed twice in the same patient, first during a phase of adrenal insufficiency (out-of-cycle) and later during a cycle of hypercortisolemia, and provide evidence that when results of IPSS are interpreted carefully in CCS, a suspected diagnostic pitfall may be avoided. Our patient’s out-of-cycle IPSS showed adrenal insufficiency due to profound suppression of the corticotrophs from the in-cycle hypercortisolism, a finding which makes the interpretation of the peak IPS:P ACTH gradient essentially non-diagnostic and should prompt further investigation during a cycle of hypercortisolism.
Very few cases of bronchial typical carcinoid producing ectopic ACTH and causing cyclical Cushing’s syndrome have been reported in the literature.10,11 To the best of our knowledge this is the first case report demonstrating proof of principle of results of IPSS when performed both in and out-of -cycle in CCS due to EAS, thereby providing a basis for a recommended approach to interpretation of results to prevent misdiagnosis of ectopic ACTH production as Cushing’s disease.
We would like to thank Dr. Lynnette Nieman and colleagues at NIH Bethesda, Maryland for their clinical involvement in the case.
A.Y.K has received funding for research from Corcept Therapeutics.

©2017 Hannoush, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.