eISSN: 2473-0815


Review Article Volume 2 Issue 3
Department of Endocrinology, Gulf Medical University, UAE
Correspondence: Saadi AlJadir, Department of Endocrinology, Gulf Medical University, PO Box 3243, Fujairah, UAE
Received: July 27, 2015 | Published: September 28, 2015
Citation: AlJadir S. Insulinoma: literature’s review (part 1). Endocrinol Metab Int J. 2015;2(3):112-122. DOI: 10.15406/emij.2015.02.00025
Insulinoma is rare a tumor of the islet cell of the pancreas that produces excessive amounts of insulin, inappropriately to plasma glucose levels. The average age of onset 40-50 years .The typical symptoms that patients complain about are related to the development of hypoglycemia. Patients with insulinoma usually develop neuroglycopenic symptoms (confusion, headache, visual difficulties, irrational behavior and extremely, coma) particularly with exercise or fasting. Severe hypoglycemia may result in seizures, coma, and eventually neurological damage. Symptoms resulting from the catecholaminergic response to hypoglycemia (i.e., tremulousness, palpitations, tachycardia, sweating, hunger, anxiety) are not uncommon. Weight gain is sometimes observed and not uncommonly the patient might become massively obese. Insulinomas are small in 90% <2cm, solitary >90 %, and 5-10% malignant. They are almost invariably occur in the pancreas and distributed equally in head, body and tail. Insulinoma should be suspected in all patients with hypoglycemia, especially those with attacks provoked by fasting or with a family history of MEN-1. The diagnosis of an insulinoma is usually made biochemically with low blood glucose, elevated insulin, C-peptide levels and proinsulin, by a 72-hour fasting under close supervision, and confirmed by localizing the tumor by computed tomography, MRI, or transabdominal and endoscopic ultrasonography. Sometimes detection of lesions might be challenging and ancillary tests are occasionally required. The definitive treatment is surgical removal of the tumor, and laparoscopic surgery for localized lesions is increasingly reported. Medical treatment is reserved for unresectable tumors, preoperative preparation or for unsuitable candidates for surgery.
Keywords: neuroendocrine, Hypoglycemia, Men-1*, islet cell tumor, whipple’s triad, insulinoma, fasting test, MRI, diazoxide
NETs, neuroendocrine tumors; GEP NETs, gastroenteropancreatic neuroendocrine tumors; PNETs, pancreatic neuroendocrine tumors; VIP, vasoactive intestinal polypeptide; ACTH, adrenocorticotropic hormone; MEN1, multiple endocrine neoplasia type 1; IGF, insulin-like growth factor; DMR2, differentially methylated region 2; ENETS, european neuroendocrine tumor society; NIPHS, non-insulinoma pancreatogenous hypoglycemia syndrome; OGTT, oral glucose tolerance test
*Multiple Endocrine Neoplasia Type 1
Insulinomas are a type of neuroendocrine tumors; they belong to a specific group of NETs called gastroenteropancreatic neuroendocrine tumors (GEP NETs). They are also called pancreatic neuroendocrine tumors (PNETs).Endocrine pancreatic tumors may be classified according to their secretory ability into functioning or non-functioning tumors. The non-functioning tumors constitute the largest group, representing ~ 50% of the endocrine pancreatic tumors; following in incidence are the insulinomas (25%) and gastrinomas (15%), while theremaining 10% include vasoactive intestinal polypeptide (VIP-oma), glucagonomas and somatostatinomas.Most of these tumors are sporadic, while about 15–30% is hereditary and appear in the context of (MEN1) or von Hippel–Lindau syndromes. Insulinomas are the most common cause of hypoglycemia resulting from endogenous hyperinsulinism. In a large single-center series of 125 patients with neuroendocrine tumors, insulinomas constituted the majority of cases (55%), followed by gastrinomas (36%), VIPomas (5%), and glucagonomas (3%).Although it is uncommoncause of hypoglycemia, but promptly comes to the mind of physicians who meet recurrent or persistent hypoglycemia in the clinical practice. However insulinoma should be put in differential diagnosis of fasting hypoglycemia in a healthy looking person. Insulinomas can be difficult to diagnose. It was not uncommon for patients to have been misdiagnosed with psychiatric illnesses or seizure disorders before insulinoma was recognized.1–13
History
The first resection of an insulinoma was performed in 1927, when WJ Mayo removed an insulin-secreting tumor from a physician and injected its extracts into rabbits that subsequently developed hypoglycemia. In 1929, Graham achieved the first surgical cure of an islet cell adenoma. In 1938, Whipple reported a triad, considered pathognomonic for insulinoma: neurologic symptoms of hypoglycemia, blood glucose levels less than 50 mg/dl and immediate alleviation of symptoms after glucose ingestion. In 1954, Wermer described a family with MEN-1 syndrome (often associated with gastrinomas and less frequently with insulinomas). In 1993, Gagner et al.,17 reported the first successful laparoscopic distal pancreatectomy and pancreatoduodenectomy. A successful laparoscopic resection for pancreatic insulinoma was made in 1996. Since then, an increasing number of reports of successful laparoscopic resection for pancreatic Insulinomas have been published in medical literatures.13–17
Pathophysiology
An insulinoma is a neuroendocrine tumor, deriving mainly from pancreatic islet cells, that secretes insulin. Some insulinomas also secrete other hormones, such as gastrin, 5-hydroxyindolic acid, adrenocorticotropic hormone (ACTH), glucagon, human chorionic gonadotropin, and somatostatin. The tumor may secrete insulin in short bursts, causing wide fluctuations in blood levels. About 90% of insulinomas are benign, the rest ˜5-10% are malignant .Around 10% of patients have multiple insulinomas; of those with multiple insulinomas, 50% have MEN-1. Insulinoma are associated with MEN1 in 5% of patients. On the other hand, 21% of patients with MEN-1 develop insulinoma during their lifetime. Because of the association of insulinomas with MEN-1, consideration should be given to screening family members of insulinoma patients for MEN-1.18–20
Frequency
United States
Insulinomas are the most common pancreatic endocrine tumors. The incidence is 3-10 cases per million people per year. These make up 55% of neuroendocrine tumors as mentioned earlier. The incidence has been reported higher in autopsy studies (0.8% to10%), suggesting that these tumors frequently remain undiagnosed.21–23
International
Exact data for international incidence of insulinomas are not available. A report Northern Ireland demonstrated an annual incidence of 1 case per million persons. A study from Iran found 68 cases in a time span of 20 years in a university in Tehran. A 10-year single-institution study from Spain of 49 consecutive patients who underwent laparoscopic surgery for neuroendocrine pancreatic tumors included 23 cases of insulinoma.21,22
Gender and Age
There is slight female preponderance; for insulinomas is 3:2 female: male ratio, and probably equal for malignant tumors. No racial difference had been reported in literatures published. The median age at diagnosis is about 47 years, except in insulinoma patients with MEN 1, in whom the median age is the mid 20s. In one series, patients with benign disease were younger (mean age of 38 y) than those with metastases (mean age of 52 y). The age range for peak incidence of insulinoma is between 30 and 60 years. The incidence of insulinomas in patients with diabetes mellitus is the same with the general population. Approximately 60 years were required to obtain accurate epidemiological data regarding insulinomas because of the rarity of these tumors (Mayo Clinic series…).12,24–28
Molecular genetics
Multiple endocrine neoplasia type 1 (MEN1) is a hereditary syndrome transmitted with an autosomal dominant trait. The disease is characterized by the occurrence of multiple endocrine tumours of the parathyroids, pancreas and anterior pituitary. Different laboratories have detected germline mutations of the MEN1 gene in about 70–90% of familial MEN1 patients. Somatic mutations were also found in a substantial proportion of several sporadic endocrine tumors, especially in insulinoma, gastrinoma and parathyroid adenoma. The mutations revealed in the above analyses showed a typical ‘loss of function’ profile, establishing no genotype–phenotype correlation. The loss of heterozygosity frequently observed in MEN1 tumors supports the hypothesis that the MEN1 gene acts as a tumor suppressor in affected cells.
To generate adequate tools for the study of the mechanisms involved in endocrine malignancy related to MEN1 gene inactivation, some researchers had generated Men1 mutant mice using either conventional or conditional gene targeting strategies. Heterozygous Men1 mutant mice start to develop the major endocrine tumors seen in MEN1 patients at around 12 months of age, whereas homozygous Men1 mutant embryos die at E11.5–E13.5 with multiple developmental defects. In parallel, pancreatic b-cell- and parathyroid-specific Men1 gene disruption results in the development of insulinoma and parathyroid adenoma respectively. They had noticed that the insulinoma started to appear in b-cell-specific Men1 mutant mice at around 6 months of age (early stage insulinoma). At 10 months, all the b-cell-specific Men1 mutant mice developed insulinomas with adenocarcinoma features (late stage insulinoma). Furthermore, mouse Men1 insulinomas in this model appeared not only earlier than those found in heterozygous Men1 mutant mice, but also relatively synchronized, making such mice a suitable model for dissecting the genetic events that occur during the tumor initiation and progression. Using this model, they had previously demonstrated the existence of a long interval period between the appearance of menin-inactivated cells and the development of insulinoma. This prompted the team to propose the hypothesis that tumorigenesis of b-cells triggered by the disruption of the Men1 gene needs the participation of other factors. To identify these factors, they carried gene expression profiling of the insulinomas developed in b-cell specific Men1 mutant mice at 6 and 10 months of age, corresponding respectively to the insulinomas at early and late stages. Their results revealed a substantial num ber of genes, whose expression is either up- or down-regulated in tumors. More importantly, these data provide evidence that the gene expression profile of the insulin-like growth factor (IGF) and cell cycle pathways is particularly deregulated. Further analysis reveals that the overexpression of IGF2 is accompanied by the hypermethylation of the intragenic DMR2 region containing elements that increase the level of transcription through methylation. They highlighted the early involvement of both genetic and epigenetic mechanisms in tumorigenesis of b cells related to MEN1 inactivation.
To gain insights into the mechanisms of the tumorigenesis related to MEN1 inactivation, reaserchers had used mice in which the Men1 gene was specifically disrupted in pancreatic b-cells. In these mice, they observed full penetrance of insulinoma with defined histological characteristics of tumorigenesis.
To identify the genetic factors taking part in the tumor development, they performed gene expression profiling analysis of these insulinomas at different stages. Here, they showed that in late stage insulinomas, 56 genes are up regulated and 194 are down-regulated more than fourfold compared with normal pancreatic islets.
Clustering analysis reveals the deregulation of Hox gene family and the genes involved in cell proliferation and cell cycle control. The altered expression of Igf2, Igfbp3 and Igfbp6 as well as cyclin A2, B2 and D2 are confirmed by quantitative RT-PCR, with the overexpression of all the three cyclins found in early stage insulinomas. Moreover, an increased proportion of cyclin A2- and D2-expressing cells and the overexpression of insulin-like growth factor 2 (IGF2) proteins are detected in mouse Men1 insulinomas by immunostaining. Interestingly, the analysis of DNA methylation patterns by quantitative serial pyrosequencing reveals that four specific CpGs in the intragenic differentially methylated region 2 (DMR2) region of the Igf2 gene known to augment transcription through methylation are significantly hypermethylated in insulinomas of Men1 b-cell mutant mice at 6 and 10 months of age, even before IGF2 overexpression can be detected. Thus, these data indicate the involvement of both genetic and epigenetic mechanisms in early tumorigenesis of b-cells related to MEN1 inactivation.29–46
Researchers had identified a gene, rig (rat insulinoma gene), that is activated in chemically induced rat insulinoma but not in normal pancreatic islets or in regenerating islets. In this study, they had found that the insulinoma gene was activated in a BK virus induced hamster insulinoma cell line and in a spontaneously occurring human insulinoma. From the hamster and human insulinoma cDNA libraries, rig homologues were isolated, and their nucleotide sequences were determined. In the same manner as the rat gene, both hamster and human homologues contained one open reading frame of 435 nucleotides, differing by 32- and 41-base substitutions, respectively. All the base substitutions were same-sense mutations. Accordingly, the deduced 145-amino acid sequence remained invariant in hamster, human, and rat insulinomas, suggesting that rig has evolved under extraordinarily strong selective constraints. Computerized structure analysis indicated that rig-encoded protein is a possible DNA-binding protein. The antisense oligodeoxyribonucleotide complementary to hamster rig mRNA was synthesized and injected into the hamster insulinoma cells. The antisense rig oligodeoxyribonucleotide inhibited DNA synthesis in the insulinoma cells, whereas the sense rig oligodeoxyribonucleotide or antisense insulin oligodeoxyribonucleotide had no inhibitory effect. These results strongly suggest that the activation of rig is both common and potentially significant in the oncogenic growth of pancreatic B cells of islets of Langerhans.47–51
General features
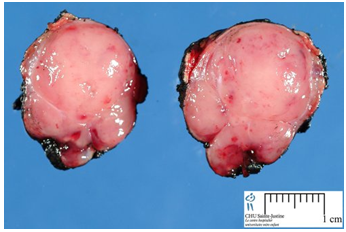
(Source: Pancreatic insulinoma: current issues and trends. Vaidakis D, Karoubalis J, Pappa T, Piaditis G, Zografos GN. Hepatobiliary Pancreat Dis Int. 2010 Jun;9(3):234-41.)
Insulinomas are rare neuroendocrine tumors with an incidence estimated at 1 to 4 new cases per million persons per year. Insulinoma is one of the most common types of tumor arising from the islets of Langerhans cells (pancreatic endocrine tumors). They comprise 70% to 80% of all functional neuroendocrine pancreatic tumors. Estimates of malignancy (metastases) range from 5% to 30%. Over 99% of insulinomas originate in the pancreas, with rare cases from ectopic pancreatic tissue. About 5% of cases are associated with tumors of the parathyroid glands and the pituitary (MEN 1) and are more likely to be multiple and malignant. Most insulinomas are small; commonly less than2cm and infrequently3cm or more (Figure 1), (Figure 2). The majority is solitary, benign lesions occurring in a sporadic setting; they are alsosingle, small, and hypervascular. Approximately 10% are multiple, 10% are malignant, and 16% are associated with MEN syndrome during lifetime. Malignant insulinomas tend to occur more frequently in MEN-1 patients. The incidence of insulinomas in patients with diabetes mellitus is the same with the general population.13,24,27,28
Malignant insulinoma
It is difficult to predict the malignant nature of an insulinoma on the basis of its histological features. The current WHO classification criteria involve the presence of metastases, gross invasion, tumorsize (larger size average 6cm), and percentage of mitoses, proliferative index and vascular invasion. However, metastases are generally considered the only definitive characteristic of malignancy. Out of allinsulinomas; ˜5%-10% are malignant. Thedistinctive diagnosis between malignant and benign insulinomas is usually very difficult and is based on intraoperative evidence (metastases in the liver, regional nodes orlocal invasion), whereas in some patients the metastases are frequently found along with the recurrence of hypoglycemic episodes.
It is prudent to discover alternative ways of estimating the malignant potentialof insulinomas and not solely rely on histopathological examination. The five-year survival rate was reported to be 60-100% for localized disease, 40% for regional disease, 29% for distant metastases, and 80% for all stages. In a publication from 1993, the 5-year survival rate for advanced EPT was approaching 60 months from diagnosis.3,52–57
Staging of tumor
Neuroendocrine neoplasms of the pancreas, also known as islet cell tumors, are relatively rare, although they are diagnosed with increasing incidence and high prevalence. Because of their overall indolent but malignant course, pancreatic neuroendocrine neoplasms have not been well studied, and standard staging tools have been lacking.
A TNM staging system for neuroendocrine neoplasms of the pancreas was proposed for the first time in the year 2006 by the European Neuroendocrine Tumor Society (ENETS TNM. More recently, the International Union for Cancer Control developed a TNM staging system, which is now endorsed by both the American Joint Cancer Committee and the World Health Organization (UICC/AJCC/WHO 2010 TNM).4–6
However, the tumor definition and derived stages of the ENETS TNM and the UICC/AJCC/WHO 2010 TNM staging systems greatly differ (Table 1).
ENETs |
AJCC |
Proposal for a TNM Classification and Disease Staging for Endocrine Tumors |
Definitions of TNM |
(T) Primary Tumor |
Primary Tumor (T) |
TX Primary tumor cannot be assessed |
Primary tumor cannot be |
T0 No evidence of primary tumor |
No evidence of primary tumor |
T1 Tumor limited to the pancreas and size <2 cm |
Tumor limited to the pancreas, £2 cm in greatest dimension |
T2 Tumor limited to the pancreas and size 2–4 cm |
Tumor limited to the pancreas, >2 cm in greatest dimension |
T3 Tumor limited to the pancreas and size >4 cm or invading duodenum or bile duct |
Tumor extends beyond the pancreas but without involvement of the celiac axis or the superior mesenteric artery |
T4 Tumor invading adjacent organs (stomach, spleen, colon and adrenal gland) or the wall of large vessels (celiac axis or superior mesenteric artery) |
Tumor involves the celiac axis or the superior mesenteric artery (unresectable primary tumor) |
Metastatic insulinoma
Insulinoma are usually benign and relatively small (<2 cm) solitary tumors; however, 5.8 to 15% of the tumors are malignant. MEN Type I is present in 7.6 to 16% of patients and is associated with a greater risk of recurrence. The 10-year survival rate is estimated at 91% and 29% for benign and malignant insulinoma, respectively.
Metastatic insulinoma is a rare disease with an indolent course. Although the disease is currently not curable, available modalities can provide long symptomatic remissions. Patients with malignant insulinoma should be followed closely, and those not responding to standard therapy should be enrolled in chemotherapy trials (Table 4) (Table 5).66–74
About 85% of patients present with symptoms of hypoglycemia that include diplopia, blurred vision, palpitations, or weakness. Other symptoms include confusion, abnormal behavior, unconsciousness, or amnesia. About 12% of patients have grand mal seizures. Adrenergic symptoms (hypoglycemia causes catecholamine’s release) include weakness, sweating, tachycardia, palpitations, and hunger. Symptoms can be divided into either adrenergic, resulting from the catecholaminergic response (anxiety, tremor, nausea, hunger, sweating and palpitations) orneuroglycopenic (headache, lethargy, dizziness, diplopia, blurred vision, amnesia, seizures and in more severe cases confusion or coma).Symptoms may be present from 1 week to as long as several decades prior to the diagnosis (1 m to 30 y, median 24 months, as found in a large series of 59 patients). Symptoms may occur most frequently at night or in the early morning hours. Hypoglycemia usually occurs several hours after a meal. In severe cases, symptoms may develop in the postprandial period. Symptoms can be aggravated by exercise, alcohol, hypocaloric diet, and treatment with sulfonylurea medications. Weight gain occurs in 20-40% of patients. Because of hyperinsulinism, or over-attempt to relieve symptoms of hypoglycemia, many patients might be eventually overweight. A case report of a patient with type 2 diabetes who developed recurrent hypoglycemia was published from France. Symptoms caused by effects of local tumor mass are very rare in insulinoma. The cardinal symptoms of insulinoma are the subsequent development of symptoms of hypoglycemia. The leading symptoms establishing the diagnosis of endogenous hyperinsulinism comprise the Whipple's triad; this includes: 1) symptoms of neuroglycopenia,2) documented hypoglycemia (plasma glucose levels<50 mg/dl), and 3) symptoms relief (often within 5-10minutes) following glucose administration. Patients with the above symptoms warrant further evaluation. Hypoglycemic symptoms tend to occur with plasma glucose concentrations at or below 50mg/dl with central nervous system disturbances becoming apparent when plasma glucose falls below 45mg/dl. It is very frequent for some patients to develop hypoglycemic unawareness, as a result of central nervous system adaptation to chronic hypoglycemia. Clinical hypoglycemic disorders have also been divided in two categories: those occurring in healthy-appearing subjects and in patients with a known illness. Because several diseases manifest with the same symptoms as hypoglycemia, plasma glucose concentrations must always be measured at the time of the episodes (Table 2A), (Table 2B) & (Table 3).75–81
Ill or Medicated Individual |
|
Drugs |
Insulin or insulin secretagogue |
Alcohol |
|
Others (see Table 2) |
|
Critical Illnesses |
Hepatic, renal, or cardiac failure |
Sepsis |
|
Inanition |
|
Hormonal Deficiency |
Cortisol |
Glucagon and epinephrine (in insulin-deficient diabetes mellitus) |
|
Non-Islet Cell Tumor |
|
Table 2A Causes of Hypoglycemia in Adults81
Seemingly Well Individual |
|
Endogenous Hyperinsulinism |
Insulinoma |
Noninsulinoma pancreatogenous hypoglycemia |
|
Post-gastric bypass hypoglycemia |
|
Autoimmune hypoglycemia |
|
Antibody to insulin |
|
Antibody to insulin receptor |
|
Insulin Secretagogues |
|
Other |
|
Accidental, Surreptitious or Malicious Hypoglycemia |
|
Table 2B Causes of Hypoglycemia in Adults 81
Moderate Quality of Evidence |
Cibenzoline |
Gatifloxacin |
|
Pentamidine |
|
Quinine |
|
Indomethacin |
|
Glucagon (during endoscopy) |
|
Low Quality of Evidence |
Chloroquineoxaline sulfonamide |
Artesunate/artemisin/artemether |
|
Insulin-like growth factor type 1 |
|
Lithium |
|
Propoxyphene/dextropropoxyphene |
|
Angiotensin-converting enzyme inhibitors |
|
Angiotensin receptor antagonists |
|
β-Adrenergic receptor antagonists |
|
Levofloxacin |
|
Mifepristone Disopyramide |
|
Trimethoprim-sulfamethoxazole |
|
Heparin |
|
6-Mercaptopurine |
|
25 Cases Identified82 |
Laboratory findings
Blood glucose levels are very tightly controlled by the pancreatic islets. Following food ingestion, blood glucose levels increase and stimulate insulin production, on the other hand when blood glucose levels decrease, insulin production is reduced. In β-cell adenomas the production of insulin is not dependent on blood glucose levels. The critical diagnostic test for insulinoma is to measure elevated serum insulin levels in the presence of hypoglycemia.
In 2009 the Endocrine Society's clinical practice guidelines for management of hypoglycemic disorders, had stated that the new cut-off of insulin for the diagnosis of insulinoma should be lowered to 3µU/mL in presence of hypoglycemia (serum glucose 55 mg/dL or less).78,82–86
Fasting test
Insulinomas are rare but are the most common cause of hyperinsulinemic hypoglycemia in the adult population. Diagnosis of this pathology relies on clinical features along with laboratory tests and imaging investigations to aid in localization. One of the most robust standard tests used for establishing a biochemical diagnosis is the prolonged (72 h) fast. Currently, it is recommended that a prolonged supervised fast be performed, at least for 48 h if not for 72 h, and many would take the absence of hypoglycemia after a 72-h fast as evidence excluding the diagnosis. Insulinoma causes fasting hypoglycemia due to inappropriate insulin secretion. Its diagnosis is based on demonstrating Whipple’s triad during a supervised 72-h fast. For 75 yr, the 72-h fast has been the cornerstone for the diagnosis; however, it has never been critically assessed using newer assays for insulin, C peptide, and proinsulin.
Some reports showed a prolonged oral glucose tolerance test clearly demonstrated the induction of severe hyperinsulinemia followed by significant hypoglycemia. They demonstrated that 72-h fast was normal in that the patients with proven insulinoma remained normoglycemic throughout; nevertheless, with a prolonged oral glucose tolerance test (oGTT) clearly demonstrated the induction of severe hyperinsulinemia followed by significant hypoglycemia. Although there are previous case reports of reactive but not fasting-induced hypoglycemia in patients with Insulinomas.
Fasting serves two purposes
First, identifying the relation between hypoglycemia and the patient's symptoms, and second, exhibiting inappropriately elevated insulin concentrations with low blood glucose levels. A 72--hour fasting (under close observation) is considered as the gold standard for the diagnosis of insulinoma; although on reality it rarely takes full 72 hours upon completion. Between 70 and 80% of patients with insulinoma will develop hypoglycemia during the first 24 hours and 98% by 48 hours. A proposed protocol for the test is the following and requires hospitalization: during the fasting period the patient is allowed to drink calorie free fluids and physical activity should be encouraged. Blood glucose levels (venous blood by standard methods) should be measured every 6 hours; when they fall to 60 mg/dl, they should be measured every 1 or 2 hours. When plasma glucose levels are 40-45 mg/dl and the patient has symptoms of hypoglycemia, blood sample should be drawn and sent to the laboratory for measurement of glucose, insulin, C-peptide (low in exogenous insulin users), proinsulin((normal in exogenous insulin/hypoglycemic agent users), some authors advocate antibodies to insulin (positive in exogenous insulin users) ,β-hydroxybutyrate, and common sulfonylureas. Surreptitious use of insulin or hypoglycemic agents may be difficult to distinguish from the symptoms of insulinomas. The new cut-off of insulin has further increased the sensitivity of 72h fasting test for the diagnosis of insulinoma from 87 to 100%. (Endocrine society 2009).
Data obtained during the supervised fast of patients with pathologically proven insulinoma over a 30-yr period (1970–2000) was reviewed. 127 patients were identified with insulinoma. The average age of patients was 42.7 yr, with a predominance of females (62%). 107 patients had a benign tumor, 20 had malignant insulinoma, and 15 patients had MEN 1. The fast was terminated due to hypoglycemia in 44 patients (42.5%) by 12h, 85 patients (66.9%) by 24h, and 120 (94.5%) by 48 h. Seven patients fasted beyond 48 h despite subtle neuroglycopenic symptoms and glucose and insulin concentrations diagnostic of insulinoma. Immunoreactive proinsulin was elevated at the beginning of the fast in 90% of 42 patients. Proinsulin in noninsulinoma, in contrast to insulinoma, patients is usually suppressible; therefore, samples taken in the suppressed state have the greatest diagnostic value. Recent workers concluded that with the current available insulin and proinsulin assays, the diagnosis of insulinoma can be made within 48 h. Thus, the 48-h fast might replace the 72-h fast in medical literatures and hospital protocols as the new diagnostic standard.
Although the sensitivity of the 72-h fast is high and still plays an important role in the diagnosis of an insulinoma, we suggest that a “normal” test result should be interpreted in the light of clinical symptoms.13,82–103
Auxiliary tests
Intravenous secretin test
In patients harboring an insulinoma, insulin production by normal pancreatic β cells is significantly suppressed. Following intravenous injection of secretin (2 units/kg of body weight), plasma insulin rises more than double in normal individuals, whereas in patients with insulinoma, the secretin stimulation test does not cause a rise in plasma insulin due to the unresponsiveness of insulinoma cells to secretin.104
C-peptide inhibition test
After proinsulin cleavage, insulin and C-peptide are secreted. Infusion of insulin for 1 hour leads to the decrease of plasma C-peptide levels in healthy subjects, but no such decrease is observed in insulinoma patients. Insulin release from insulinoma cells is not inhibited by the administration of exogenous insulin, whereas insulin secretion from normal β-cells is inhibited by the increased plasma insulin.105
Functional localization
Selective pancreatic intra-arterial calcium injections to localize islet cell tumors were introduced in the1980s. This technique can be used in as many patients as feasible, regardless of the results of cross sectional imaging, to provide a corroborative functional perspective. A number of series have reported the additional value of this test in localization, particularly in cases where non-invasive methods have been unsuccessful. The test offers a high sensitivity, and if coupled with angiography might be 95%.106–108
Differential diagnosis
In the emergency ward two thirds of the patients with hypoglycemia have DM and nearly one fourth areseptic. Drugs, mainly insulin, are the most common cause of hypoglycemia in hospitalized patients. Critical illness, renal or hepatic failure and sepsis are also common, whereas hypoglycemia due to hypocortisolismis an uncommon etiology. Clinical manifestations of hypoglycemia vary among patients. The causes of hypoglycemia in healthy appearing patients include insulinoma, non-insulinoma pancreatogenoushypoglycemia syndrome (NIPHS), insulinfactitious hypoglycemia, strenuous exercise, and ketotichypoglycemia, with insulinomas being the largemajority of these cases. Although it requires several days of hospitalization, the diagnosis of insulinoma can be established according to the following criteria;(1) blood glucose levels near or below 45 mg/dl with hypoglycemic symptoms; (2) inappropriately high insulin levels (for some author over 6 μIU/ml0),(3) elevated C-peptide levels(over 0.2 nmol/L),plasma proinsulin concentrations of at least (5.0 pmol/L), and (4)measurement of a plasma or urine of circulating oral hypoglycemic agent (ideally allsulphonylureas & glinides)screen to exclude surreptitious hypoglycemia and the abuse use of such medications, although not all oral hypoglycemic agents, for example repaglinide, can be detected in this way).106,109–115
The combination of biphasic thin section helical CT scan and EUS has an almost 100% sensitivity in localizing insulinomas. Laparoscopic US are mandatory for intraoperative localization of these tumors. EUS-guided fine needle tattooing represents an alternative method, when laparoscopic US is not available. Laparoscopic resection of benign insulinomas is the procedure of choice, whereas pancreatectomies are reserved for the rare cases of large, potentially malignant tumors.
We conclude that insulinoma is less rare than previously suspected. After successful surgical removal, the long-term risk of recurrent insulinoma is relatively high in patients with MEN I; for patients with benign disease, the long-term survival is normal.
None.
The author declares that there are no conflicts of interest.

©2015 AlJadir. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.