eISSN: 2473-0815


Research Article Volume 12 Issue 4
State Scientific Center of the Russian Federation, Institute of Biomedical Problems of the Russian Academy of Sciences, Russia
Correspondence: Yuri A Koryak, State Scientific Center of the Russian Federation, Institute of Biomedical Problems of the Russian Academy of Sciences, Moscow, Russia, Tel +7910 457 43 21
Received: November 10, 2024 | Published: December 11, 2024
Citation: Koryak YA. Influence of menstrual cycle on isometric strength, muscle architecture, and biomechanical efficiency of lower limb extensor muscles in girls after using 3-day ‘dry’ water immersion model. Endocrinol Metab Int J. 2024;12(4):112-126. DOI: 10.15406/emij.2024.12.00357
Purpose: The present study aimed to investigate the changes in the muscle architecture (lengths and angles of fascicles) of the female medial gastrocnemius muscle (MG) in vivo and investigate changing biomechanical properties of muscles with increasing drop jump height during the menstrual cycle after 3 days of ‘dry’ water immersion (DI).Methods: Six female subjects with moderate physical activity level and regular menstrual cycles volunteered to participate in the study. The subjects were young healthy girls (n = 6, age = 28.4 ± 1.4 yr, height = 1.7 ± 0.1 m, mass = 60.2 ± 3.8 kg) with a normal menstrual cycle lasting between 26 and 32 days (mean, 28) and not taking any form of hormonal treatment. The maximum voluntary isometric contraction (MVC) of the plantar flexors muscles was measured using an isokinetic dynamometer (Biodex, USA) at ankle angles of 0o. The subjects performed the drop jumps (DJ) from 20 cm (DJ20), 40 cm (DJ40) and 60 cm (DJ60) on a contact plate. Statistical comparisons of vertical ground reaction force (vGRF), impulse, moment, power, and work were made between different drop jump heights. A real-time B-mode ultrasound apparatus (Edge, USA) and a linear 7.5-MHz electronic transducer with a 60-mm field of view were used to obtain images of the human MG (lengths and angles of fascicles) at rest and during MVC (100%). Additional images were taken at 20 and 80% of MVC at an ankle angle of 0o. The internal architecture of the MG was determined at ankle angles of -15o (dorsiflexion), 0o (neutral anatomical position), and +30o (plantarflexion) with an angle in the knee joint of 0o. In each position, longitudinal ultrasound images (US) of MG were obtained at the proximal levels 30% of the distance between the popliteal crease and the center of the lateral malleolus. US images were obtained at rest for each ankle position, and the fiber length (Lf) and pennation angle relative to the aponeurosis were determined. Cycle phases were divided into the menstrual (when estradiol concentrations were low), follicular (when estradiol was elevated). The subjects performed three sets of experiments (‘Isokinez’, ‘Jumping’, and ‘Architecture’). In the late follicular phase were performs the campaign consisted of 2 days of baseline data collection (BDC). The day before the start of the subject's exposure to the DI conditions, the experiment ‘Isokinez’ and ‘Jumping’ was performed (BDC-1) and the next day after the rise [rise (R); R +1] from the bath. Experiment "Architecture" was performed directly on the day of exposure (BDC-0) of the subject and immediately after getting up (R +0; later follicular phase) from the bath.Results: Significant changes in MVC, muscle architecture, and mechanical properties of human muscle were found during the late follicular phase the menstrual cycle. There was a significant increase in MVC (about 6%) during later follicular phase menstrual cycle. A greater DJ height performance was observed during the later follicular phase compared to the early follicular phase.Conclusion: These results suggested that the changes in female steroid hormones during the menstrual cycle increases the muscle strength and did affect the mechanical properties of human muscle in follicular phase menstrual cycle. Thus, provide support for the influence of these hormones in regulation of these parameters in under conditions of unloading of the muscular apparatus
Keywords: unloading, pennate muscle, muscle fascicle and angles behavior, ultrasonography, drop jumps, voluntary contractions
ACSM: American college of sports medicine; BDC: baseline data collection; DI: ‘Dry’ water immersion; DJ: drop jumps; DJ20 - DJ60; drop heights; Lf: lengths of fascicles; : angles of fascicles; MVC: maximal voluntary contraction; MG: medial gastrocnemius; PCSA: index physiological cross-sectional area index; R: rise ±SE standard errors; SSC: stretch-shortening cycle; Tm: thickness muscle.
At present; the participation of women in manned space flights has increased. So; if; as of June 2013; demographic data on the international population of astronauts and cosmonauts showed that a total of 534 people flew into space - 477 men and 57 women;1 then as of May 2023 out of 634 already2 73 space travelers were women. Therefore; to ensure the safety of female astronauts (cosmonauts) during long-term space flights; it is necessary to study and understand mechanisms the changes in the contractile properties on the skeletal muscles. The study of the contractile function of the skeletal muscles of women under the influence of space flight factors is becoming increasingly important. On Earth; bed rest studies with a 6° head down are the accepted counterpart to real-world long-term microgravity used for musculoskeletal studies. The model has been successfully used in studies with the participation of men as subjects3–7 as well as in groups of subjects without taking into account gender differences.4,6,8–12 In the latter case, apparently, the researchers proceeded from the fact that traditionally, adaptations to training and reactions to physical activity were considered the same in both men and women.13,14 However, a growing body of evidence14,15 points to cyclic changes in steroid hormone levels (eg:estrogen) during the ovulatory menstrual cycle. The menstrual cycle is a complex physiological phenomenon and females, physical and physiological changes.16 The decrease in muscle strength during menstruation was first reported over 50 years ago.17 Another model that reproduces the conditions of unloading this ‘dry’ water immersion (DI).18 Surprisingly, we did not find in the literature studies involving women as subjects on the effect of the (DI) on contractile properties and muscle architectonics. We understand that it is extremely difficult for women to participate in the DI model, given the physiological characteristics of the female body. Nevertheless, it is critical to determine whether the menstrual cycle affects muscle strength, internal structure, and mechanical properties of muscles in order to plan more effective training and rehabilitation protocols for women after a long space flight.
It is well known that men and women differ in many aspects of the musculoskeletal system, with men generally having greater muscle mass and strength. In this regard, an important question arises: space flight of the same duration is accompanied by an equal loss of muscle strength in men and women. If this is indeed the case, then it is a strong argument in favor of the development of separate preventive measures for women and men. This is an extremely important problem. However, along with this, it is worth noting another and no less important problem, based on the differences in the physiological functions of the body of men and women, which must be taken into account in the training process.
Understanding the physiological effects of sex hormones on different systems of the body is crucial for sound scientific design.13 Fluctuations in some of the physiological parameters of various systems may be related to oscillations in the hormonal levels found in different phases of the menstrual cycle. Variations in the secretions of sex hormones during the different phases of menstruation may have variation in their physical performances. These oscillations seen in female steroid hormonal levels do affect the motor functions6,19 Fluctuations in estrogen potentially affect physical performance of females.20 The female sex hormones apart from their reproductive function affects various physiological systems and they have a role seen in association with exercise performance.21 Its phases are influenced by alteration in the concentration of hormones such as estrogen and progesterone.22,23 Certain physiological parameters such as athletic performance could change along with the phases of menstrual cycle.24 Fluctuating levels of sex steroids across normal menstrual cycle affect sensory-motor association of an individual.25 Maximal muscle strength appears greater in the follicular phase and at ovulation than in the luteal phase both in untrained26,27 and trained females28 and recovery and reconstruction of muscle fibers have been shown to be faster in the mid follicular phase than the luteal phase after eccentric exercise.29,30 Thus confirming that estrogen does exert a positive effect on skeletal muscle. Furthermore, no studies have been done on the influence of menstrual cycle on the mechanical properties of muscle of female in the DI.
Vertical jumping has been described as a complex human movement that requires a high degree of motor coordination between upper- and lower body segments.31 The vertical jump is often used as a performance test to assess man ability, identify athletes’ strengths and weaknesses, and measure the effectiveness of training programs.32 The maximum jump height achieved by an individual, which is an indicator of leg muscular power, can provide key information about their functional capacity and performance.33 In fact, vertical jumping is considered an essential indicator of functional performance of the neuromuscular system in human. The vertical jump performance is determined by a complex interaction among several factors, including the maximal force developed by the musculature involved, the rate at which force can be developed, and the neuromuscular coordination of the upper- and lower-body segments.21,34 To measure the contribution of these factors, different devices have been used, including the use of contact mats, and force plates.35 Common performance measures calculated from these devices are flight time, vertical ground reaction force, the vertical velocity at take-off and peak power.
The architecture of a skeletal muscle is an important determinant of its functional characteristics.36–38 Muscle strength and power are the determinants of the successful performance of a motor task. Gravitational loading appears to be necessary for the maintenance of human lower limb skeletal muscle size and force.39,40 A decrease or complete absence of muscle loading leads to a decrease in the function and size of skeletal muscles.11,40 Greater loss in muscle strength than in mass is the best-known phenomenon in the effect of actual (a space flight) or simulated (prolonged bed rest) microgravity.41 The phenomenon directly indicates that factors other than atrophy additionally contribute to muscle weakness. Muscle architecture has been known to be associated with performance.42 Increases in fascicle thickness are highly correlated with ability to produce force.43,44 Increase in pennation allows for a greater number of fibers to be present within a given cross-sectional area and thus is often associated with increased muscle and fascicle thickness and increased strength.5 However, when cross-sectional area is constant but angle of pennation differs, a deficit in strength can be observed along with an increase in pennation angle.45 This is because that angle of pull of the fibers is indirect to the pull of the muscle in total, and thus the pull of the muscle in total is diminished by cosine of the pennation angle. Fascicle length is a product of both pennation angle and fascicle thickness because as pennation angle decreases or fascicle thickness increases, fascicle length will also increase. A greater fascicle length represents either longer sarcomeres or more sarcomeres in line. Thus, as the length of the contractile element increases, so does the velocity of contraction and the force that can be applied at a high velocity.
In this report, we have studied by means of ultrasonography the relationships between muscle architecture (lengths and angles of fascicles) of human MG muscles in vivo in passive (relaxed) and active (contracting) conditions, and force contractions. Therefore, information regarding skeletal muscle function during simulated microgravity may help elucidate the etiology of decrease force during spaceflight. In view of this, the objectives of the study were, first, to investigate the changes in internal architecture as a function of the ankle joint angle for the medial gastrocnemius muscle (MG) and, secondly, evaluate the change mechanical efficiency of lower limb extension muscles during vertical jumping drop jump (DJ) in young female after the DI. Taken altogether, the purpose of the purpose of the present study was to investigate how the main determinants of maximum voluntary contraction muscle in young healthy female are affected by 3 days of the DI during the menstrual cycle. In addition, we aimed of this study was to investigate how biomechanical properties change with increasing the DJ heights. We hypothesized that the kinematic and kinetic parameter in vertical jumps would be reduced following unloading in the late follicular phase.
Overall study design
The overall design of this investigation integrated several experiments that examined the physiologic, responses to simulated weightlessness (i.e. 3-day the DI) in young healthy females. In view of this, the objectives of the study were, first, to investigate the changes in internal architecture as a function of the ankle joint angle for of the triceps surae complex (medial gastrocnemius – MG) and, secondly, evaluate the change mechanical efficiency of lower limb extension muscles during DJ in young female after the DI. The study was conducted at the IBMP RAS in Moscow (Russia).
The study was conducted in the, Institute of Biomedical Problems of the Russian Academy of Sciences after obtaining Ethical clearance certificate from the Bioethical Commission of the Institute of Biomedical Problems of the Russian Academy of Sciences (Protocol No. 544 of July 16, 2020) and in accordance with the latest update of the Declaration of Helsinki (2013). All participants voluntary provided their written informed consent after having the potential risks, benefits and nature of the upcoming study explained.
Six healthy young females-volunteers aged between 24 and 39 years who were menstruating regularly were recruited through local advertisement and the subjects had never been subjected to microgravity model studies. Descriptive physiological data are reported in Table 1. All participants had a regular menstrual cycle for the four months previous to the experiment (28 ± 2 days). During a prestudy physical examination and none had any evidence of menstrual disorders. They did not take oral contraceptives or other hormones for at least 6 months before entering or during the study. Subject characteristics are presented in Table 1.
|
Age, yr |
Height, cm |
Weight, kg |
BMI, kg/m2 |
|||
|
Before |
After |
Before |
After |
Before |
After |
|
|
30,2 |
166,3 |
166,5 |
62,1 |
60,5 |
22,4 |
21,8 |
|
±2,1 |
±3,1 |
±3,1 |
±3,1 |
±3,2 |
±0,8 |
±0,9 |
Table 1 Anthropometric data for subjects (n = 6)
+-body mass index
Participants were not using any form of hormonal contraception for at least the six months previous to the experiment. Furthermore, all participants were free from any type of menstrual disorders (e.g., dysmenorrhea, amenorrhea, or heavy symptoms associated with pre-menstrual syndrome), had no musculoskeletal injuries in the three months previous to the investigation and were not taking drugs or dietary supplements during the duration of the experiment. Selection of subjects was based on a screening evaluation that consisted of a detailed medical history, complete blood count, urine analysis, resting electrocardiogram, and a panel of blood chemistry analysis, which included fasting blood glucose, blood urea nitrogen, creatinine, lactic dehydrogenase, bilirubin, uric acid, and cholesterol.
All of the subjects were considered to be in good physical condition. No subject was taking medication at the time of the study, and all subjects were nonsmokers and with no neurological disorders participated. Two weeks before the onset of the experiment protocol, informed consent was obtained from each participant.
The subjects performed there sets of experiments (‘Isokinez’, ‘Jumping’, and ‘Architecture’). The campaign consisted of 2 days of baseline data collection (BDC). The day before the start of the subject's exposure to the DI conditions, the experiment ‘Isokinez’ and ‘Jumping’ was performed (BDC -1) and the next day after the rise [rise (R), R +1] from the bath. Experiment "Architecture" was performed directly on the day of exposure (BDC -0) of the subject and immediately after getting up (R +0) from the bath. The subjects were familiarized with the experiment during a preliminary session before starting the tests. Each subject served as his own control.
The subjects measured their basal body temperature orally for 20 seconds, immediately upon awakening in the morning, using a digital thermometer (C-531, TERUMO Co. Ltd., Tokyo, Japan) with scale steps of 0.018 C. Basal body temperatures were recorded in a logbook. We confirmed the biphasic characteristics of basal body temperature.
‘Dry’ water immersion
‘Dry’ water immersion (DI) was used to simulate microgravity as described by Shulzhenko & Vil-Villiams.18 Each subject was positioned horizontally in а special bath оn fabric film that separated him from the water. During DI, the subjects remained in a horizontal position (a angle which make the body and horizontal line, e.g. 5 o head-up position) continuously for all including excretory function and eating. The water temperature was constant (33.4± 2oC) and maintained automatically at this level throughout the experiment. The duration of the DI was 3 days. The subjects were allowed to leave the immersion bath for some hygiene procedures and examinations. The average time spent by the subjects outside the immersion bath was ~ 28 min, 22 and 18 min on the first, the second and third days, respectively. Furthermore every evening, the subjects were raised from the bath for an average of 15–20 min to carry out hygiene procedures, and most of which were performed in the supine position. А nursing staff was present for subjects' transportation, maintenance of hygiene including toilet, provision of food and medical care, as well as support of subjects' needs within the constraints of the protocol. Subjects were under medical observation for
Measurement of maximal voluntary isokinetic contraction
Before starting muscle strength measurements, subjects lay back in a supine position to reduce the effect of prior activity, as an exogenous factor.46 All subjects were instructed to abstain from food for 2 hours before testing, from caffeine for 4 hours before testing, and from exercise for 12 hours before testing. Standard joint-specific warm-up procedures were followed and consisted of five submaximal repetitions and two to three maximal repetitions. After the warm-up, subjects rested at least 2 min. Strength tests were performed such that subjects exerted a maximal effort in only one direction for each set of repetitions.
After a full warm-up, isometric ankle extension torque of the subjects’ right legs was tested at joint angular velocities of using a Isokinetic Dynamometer Biodex (Biodex System 4 PRO™, Biodex Medical Systems, Shirley, New-York, USA). Participants were seated upright in the Biodex dynamometer chair with their trunk positioned and secured to the seatback with waist and shoulder belts to ensure consistent positioning and minimal movement. The hips were positioned to 90 o with the thigh and the knee angles at 45 o flexed and the ankle was in the 90° joint angle were taken to determine the plantarflexion torque. The lateral malleolus of the right foot was aligned with the axis of rotation on the Biodex dynamometer. The foot was fastened to the footplate with inelastic straps that were firmly secured behind and on the underside of the footplate to prevent heel lift. When heel lift occurred or torque did not return to baseline protocol was stopped and repeated after 3–5-min rest. Subjects performed three sets of four repetitions of maximal isometric ankle extension at an angular velocity of with a 2-min resting period between contractions unless the third trial exceeded one of the two first ones by more than 10 %. In that case an additional trial was performed. The participants were instructed to grip the side handles to help stabilize the trunk.
Each subject was instructed to exert maximal effort in only one direction and in every movement when performing a test, no verbal instruction was provided during testing. Two min rest separated the sets. Isometric of peak torque at (maximal voluntary contraction – MVC) were recorded for each subject. The contractile properties of muscles were determined 2 days before the start of immersion and 1 day after the end of immersion.
Jump test
The detection of jump heights and contact times of the jumps described below was carried out using a contact mat measuring system (Ergojump-Skurvydas, Lithuanian Sports University Kaunas, Lithuania).
Drop jump
A second testing session was performed by subjects within 24 hours of the first. The drop-jump of custom-built steel staircase with step dimensions was used to collect the fast-reactive strength. Six young healthy females performed a vertical jumping test consisting of vertical jumps type dropping (DJ) from heights of 20 (DJ20), and 40 (DJ40), and 60 (DJ60) cm. subjects landed on both feet over the contact platform (70 x 70 cm), which was placed approximately 20 cm in front of the custom-built steel staircase edge. To ensure safety of participants during the DJ landing phase, the force platform were positioned within a heavy-duty foam surr (40 cm x 40 cm). In three jumps subjects were asked to immediately jump off the ground after landing with maximum effort and jump as high as possible.47,48 In the starting position, the subjects stand upright on a custom-built steel staircase. Hands were placed on waists to restrict arm movement during the DJ. The subjects were allowed to practice several times before data collection. As with the first testing session, subjects were required to refrain from working out for at least 24 hours. Before testing, the subjects all performed a standardized warm-up consisting of dynamic lower-body stretches. No static stretching was done because of its known possible interference with power production and the disruption of the elastic component. The dynamic stretches included body weight squats, knee hugs, walking lunges, walking quadriceps stretch, high kicks, and lateral lunges.
All 3 jump types were performed in 2 consecutive sets of 3 jumps in set in both jumps, the instructions were to jump as high as possible. The subjects were allowed to practice several times before data collection. Subjects rested 3 minutes between sets to allow complete recovery 28. Subjects were told to drop to the most natural position before jumping. All jumps were performed with the hands on the waist to limit any upper body influence on the jumps.
All jumps were performed on a calibrated contact platform.35 From this position, the subject starts with a forward swing of a leg from the steel staircase and aims to jump as high as possible after a short contact with the ground, paying attention to a bouncing jump execution. Ground contact times of ≥ 200 ms, disengagement of the hands from the hips, ground contact of the heels during the takeoff or excessive joint angle enlargement in the knee and hip joint, lead to an invalid jump. Subjects received verbal encouragement during testing, which remained consistent between all subjects and trials.
A contact platform with a set electronic timer was synchronized to a vertical jump meter of measuring the flight time of the different jumps (H-Matis, Institute of Sports, and Kaunas, Lithuania). The time onset was triggered by the unloading of the subject’s feet from the platform and was stopped at the moment of touch down. This method assumes that the position of the jumper on the mat is the same in take-off and landing. The best performance in jump height was used for further analysis. When performing jumping exercises, the flight time, the height of the jump, the force (‘explosive’ force) and the time of repulsion from the platform were determined. The flight time measured from the sound signal was used for calculating the height of the rise of the center of gravity h in each jump. The rise in the body’s centre of mass above the ground (h in meters) was automatically calculated by the H-Matis from the flight time (tf in seconds) applying the following formula, where g is the acceleration due to gravity 49: h = tf2 x (g x 8-1)
Vertical velocity the rise in the body’s centre of mass above the ground was calculated from the ground contact time (tc in seconds) applying the following formula, where g is the acceleration due to gravity (9.81 m x s-2): =
A subject was seated as convenient in a chair of the Biodex isokinetic dynamometer. The trunk was fixed to the back of the chair with shoulder and waist belts to ensure a constant position and minimal movements. The hip joint was fixed relatively rigidly at an angle of 120°, the knee joint, at an angle of ~90°, and the ankle joint, at a plantarflexion angle of 20°. The configuration of joint positions was explained by the fact that the gastrocnemius muscle–tendon complex is relatively weak when the knee angle is 90°, and a 20° plantarflexion angle was used to compensate for this effect at the ankle joint. The lateral malleolus of the right foot was brought into coincidence with the rotation axis of the isokinetic dynamometer, and the center of the knee joint was thoroughly aligned with the rotation axis of the detector of the dynamometer. The subject was allowed to hold side handles to stabilize the trunk position. Each subject’s right foot was relatively rigidly fixed on a special platform he Biodex isokinetic dynamometer, which allowed the ankle joint angle to be set passively at –15o (plantar flexion), 0o (a neutral position), +15o, and +30o (plantar extension).
Real-time evaluation of the MG architecture in vivo at rest was performed using ultrasound scanner (Edge, SonoSite, USA) with a 7.5-MHz linear electronic transducer in the B mode. To improve acoustic coupling, special gel was applied onto the scanning surface of the transducer and the skin over the target muscle, the transducer was oriented along the middle sagittal axis of the muscle. The transducer was arranged along the plane of muscle bundles so that all bundles visible in the scanning window were accessible for examination. Measurements were performed for a part of each bundle visualized in the scanning window, and the invisible part was evaluated via linear extrapolation of bundles and aponeuroses. The measurement error was ~ 4 % with this approach.50 The lengths and pennation angle of muscle fibers were measured on the images. In each angle position, longitudinal ultrasonic images of the triceps surae [medial (MG) gastrocnemius muscle were obtained at the proximal levels 30 % of the distance between the popliteal crease and the center of the lateral malleolus.51 Each level is where the anatomic cross-sectional area of the respective muscle is maximal.52 At that level, mediolateral widths of MG were determined over the skin surface, and the position of one-half of the width was used as a measurement site for each muscle. The scanning head was coated with water-soluble transmission gel which provided acoustic contact without depressing the dermal surface. The transducer pressure on the skin was kept minimal during scanning in order to avoid pressing the muscle. By visualizing the fascicles along their lengths from the superficial to the deep aponeuroses, one can be convinced that the plane of the ultra-sonogram is parallel to the fascicles,53 otherwise, the fascicle length would be overestimated and the fascicle angle would be underestimated.54 The echoes from interspaces of the fascicles were sometimes imaged more clearly along the length of fascicles when the plane was changed slightly diagonally to the longitudinal line of each muscle, in which case the recreated image was used. The images were saved on the hard disk to obtain files for further analysis. The participants of the experiment was instructed to relax ankle extensor muscles during measurement, and the fiber length and angle were measured 20 min after to allow the bodily liquid medium to achieve a steady state.55,56
The length of fascicles (Lf) across the deep and superficial aponeurosis was measured as a straight line (Figure 1).56–58 To determine Lf, the visible part of fibers was measured directly in a ultrasound scanning window. A minor part of the bundle was outside of the window in some cases and was therefore estimated via linear extrapolation of fibers and aponeuroses.59 The method adequately measures the muscle fiber (bundle) length60 and is highly reliable, showing a within-class correlation coefficient of 0.99. A linear extrapolation error is no more than 2–7%.48
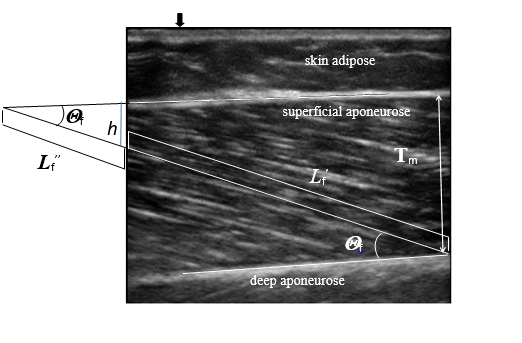
Figure 1 A standard ultrasound image of a muscle.
Sagittal ultrasound image of the medial gastrocnemius (MG). The ultrasound transducer was placed over the muscle at the level of 30 % of the distance between the popliteal fold and the center of the lateral malleolus. The pennation angle
was measured as an angle of the line drawn parallel to a fiber between the deep and superficial aponeuroses. A diagonal white line superimposed on the ultrasound image shows the path of a fiber between the superficial and deep aponeuroses. The fiber length (Lf) was measured along an ultrasound signal line drawn parallel to a fiber between the deep and superficial aponeuroses (horizontal white line). When the end of the fascicle extended off the acquired ultrasound image, fascicle length
was estimated by trigonometry
by assuming a linear continuation of the fascicles. Muscle thickness (Tm) was obtained by measuring the distance between the superficial and deep aponeuroses. A hyperechoic marker
was placed between the skin and the ultrasonic probe as the landmark to confirm that the probe did not move during measurements.
The fascicle pennation angle was measured from the angles between the echo of the deep aponeurosis of each muscle and interspaces among the fascicles of that muscle (Figure 1).56,58,61
The distance between aponeuroses (muscle thickness oTm) was estimated from the fascicle length and pennation angle using the following equation:
, where
Lf and α is the pennation angle of each muscle determined by ultrasound.
In the present study, ultrasonic measurement was repeated three times for each individual and averaged values were used. The coefficients of variation of three measurements were in the range of 0–2 %. The physiological cross-sectional area (PCSA) was estimated in the present study by the following equation48:
Conventional statistical methods were used for the calculation of means and standard errors (±SE). Differences between baseline (background) values of the subject and those post exposure were tested for significance by Student’s paired t-test and Wilcoxon non-paired test. Values are given as means ± SE in the text. Significant differences between means were set at the P < 0·05 level. The percentage changes for pre- and post-exposure were calculated.
All the women belonging to the age group 24 to 39 years with mean age 30.2 ±2.1 and have before unloading BMI 22.4 ± 0.8 kg/m2 and after 3-day unloading 21.8 ± 0.9 kg/m2 (Table 1). All the subjects have vital parameters within range. A baseline evaluation showed that contractile properties of the female muscle extensors of the foot were within the physiologically normal ranges and the subjects could be characterized by their functional potential as healthy people who lacked neuromuscular disorders and had a common lifestyle with respect to exercise. A post-3-day unloading showed changes in both contractile functions of the muscle extensors of the foot and internal architecture of the MG.
Plantar flexor torque
The changes maximal voluntary strength in plantar flexion torque under long-duration 3-day unloading is shown in Figure 2 (left panel) and reveals a significant increase. Torque increased from 106,2o 3,1 N to 112,4o 5,2 N at 0° /s-1 following 3-day unloading (p <0.05) corresponding to a relative change of 5.8 %.
Jump performance
The Drop Jump (DJ) test with a dropping height of 20 cm, 40 cm and 60 cm showed all participants. Figure 3 gives the jump height, the flight time, the vertical ground reaction force, the ground contact time, the peak vertical ground reaction force, the vertical take-off velocity during maximal jumps. Differences were found between days BDC-1 and R+1. There were found significant time effects in the jump height, the flight time, the vertical ground reaction force, the ground contact time, the peak vertical ground reaction force, the vertical take-off velocity. On day R+1, the jump height was increased from 25± 2 cm to 27± 1.4 cm corresponding to a relative change of 8 %, flight time from 450± 20 ms to 470± 10 ms, corresponding to a relative change of 4,4 %, ground contact time from 360± 50 ms to 490± 80 ms, vertical take-off velocity from 2,20± 9 m/s to 2,31± 5 m/s corresponding to a relative change of 5 % and peak vertical ground reaction force no significant was changes in DJ20 cm (Figure 3, top panel).
At the same time, in DJ60 cm the jump height, the flight time, the ground contact time, the vertical take-off velocity during maximal jumps increased by 12 %, 7 %, 6 %, 17 %, respectively, and peak vertical ground reaction force no significant was changes (Figure 3, lower panel).
Architectural characteristics before unloading
Architectural characteristics at rest
Tm of MG at rest (~14 mm) did not change significantly in response to changes in muscle length resulting from changes in ankle joint angle (Fig. 4). In muscle at rest, and Lf were ankle angle dependent. In muscle, as ankle angle increased from -15o to +30o, the increased from 18,1 ± 2,0 to 25,5 ± 1,7 deg (40,9 %, p < 0·01). In muscle, as ankle angle increased from -15o to +30o, Lf decreased in MG from 38,2 ± 2,1 to 34 ± 1,8 mm (11 %, p < 0·01).
Architectural characteristics during MVC
Tm of MG during MVC (~16 mm) did not change significantly in response to changes in muscle length (Fig. 4). In muscle during MVC, and Lf were ankle angle dependent. In muscle, as ankle angle increased by 32o (when -15o, 63,9 %, р < 0·01), by 27,6o (when +15o, 55.1 %, р < 0·01) and by 24,6o (when +30o, 49,1 %, р < 0·01). In muscle, as ankle angle increased from -15 to +30 deg, fiber length decreased by 16 mm (when -15o, 72 %, р < 0,·01), by 13 mm (when +15o, 60 %, р < 0·01) and by 11 mm (when +30o, 50 %, р < 0·01), respectively. Tm of MG (~16 mm) at any given ankle angle was not significantly different between rest and MVC.
Architectural characteristics during graded isometric force
During graded isometric force up to 100% of MVC at the neutral ankle position, the thickness of MG remained constant (Figure 4). increased and Lf decreased as a function of contraction intensity in muscles. in MG gradually increased by 22o (44,7 % when 20 %, р < 0·01), by 20o (40,1 %, when 40 %, р < 0·01), by 21o (41,1 %, when 60 %, р < 0·01) and by 13o (25,1 %, when 80 %, р < 0·01), respectively. Fibre length in MG decreased gradually by 8.8 mm (39,6 %, when 20 %, р < 0·01), by 5,2 mm (23,4 %, 40 %, р < 0·01), by 2,7 mm (12,2 %, 60 %, р < 0·01) and by 0,8 mm (3,6 %, 80 %, р < 0·01), respectively.
Architectural characteristics after unloading
Architectural characteristics at rest
Tm of MG at rest (~14 mm) did not change significantly in response to changes in muscle length resulting from changes in ankle joint angle (Figure 4). In muscle at rest, Lf were ankle angle dependent. In muscle, as ankle angle increased from -15o to +30o, the increased from 19,0 ± 2,0 to 28,5 ± 1,7 deg (50 %, p < 0·01). In muscle, as ankle angle increased from -15o to +30o, Lf decreased in MG from 37,3 ± 2,1 to 24,9 ± 1,8 mm (33,3 %, p < 0·01).
Architectural characteristics during MVC
Tm of MG during MVC (~16 mm) did not change significantly in response to changes in muscle length (Figure 4). In muscle during MVC, of and Lf were ankle dependent. In muscle, as ankle angle increased from -15o to +30o, the of increased by 23o (when -15o, 54,3 %, р < 0·01), by 18,7o (when +15o, 45.1 %, р < 0·01) and by 13,4o (when +30o, 32,2 %, р < 0·01), In muscles, as ankle angle increased from -15 to +30 deg, Lf decreased by 18,4 mm (when -15o, 72 %, р < 0,·01), by 13 mm (when +15o, 60 %, р < 0·01) and by 11 mm (when +30o, 50 %, р < 0·01), respectively. Tm MG (~14 mm) at any given ankle angle was not significantly different between rest and MVC.
Architectural characteristics during graded isometric force
During graded isometric force up to 100 % of MVC at the neutral ankle position, the thickness of MG remained constant (Figure 4). increased and Lf decreased as a function of contraction intensity in muscles. in MG gradually increased by 22o (44,7 % when 20 %, р < 0·01), by 20o (40,1 %, when 40 %, р < 0·01), by 21o (41,1 %, when 60 %, р < 0·01) and by 13o (25,1 %, when 80 %, р < 0·01), respectively. Lf in MG decreased gradually by 8.8 mm (39,6 %, when 20 %, р < 0·01), by 5,2 mm (23,4 %, when 40 %, р < 0·01), by 2,7 mm (12,2 %, when 60 %, р < 0·01) and by 0,8 mm (3,6 %, when 80 %, р < 0·01), respectively.
The present study was planned as a study of the degree of adaptation of the contractile functions of the foot extensor muscles (TS) and the internal architecture of the MG, as one of the three heads of the TS complex that contributes to the generation of MVC, in a 3-day unloading (DI) in a group of healthy young girls with regular menstruation. For the first time, the architecture and biomechanical properties of the muscles of the lower extremities were studied during the performance of vertical jumps in girls under conditions of a 3-day DI. The main results show that prior unloading resulted in changes in muscle contractility and muscle architecture. This is the first study to describe the architecture of human MG under in vivo conditions at rest and during isometric plantar flexion to full contractile state (MVC) after 3-days of unloading. The study showed the reconstruction of the architecture of one of the main locomotor muscles caused by unloading. The main conclusion of this study is that, firstly, the architecture of the MG (lengths and angles of fascicles) changes in response to changes in the position of the ankle joint and the developed force. Secondly, the MG thickness did not change depending on the intensity of muscle contraction, and thirdly, the unloading of the muscular apparatus caused by 3-day unloading under DI conditions was accompanied by an increase in the maximum voluntary torque moment (MVC) and the biomechanical properties of the muscles lower limbs during vertical jump. Contrary to our expectations, the isometric MVC of the foot extensor muscles after 3 days of unloading not only did not decrease, but even increased. It is well known that prolonged mechanical unloading of muscles leads to significant maladjustment of the musculoskeletal system.62 However, in the present study, changes in maximal foot extensor muscle activation appear to be in conflict with the aforementioned disuse paradigms. The main finding confirms our initial hypothesis, as the results of explosive movements (i.e. jumps requiring the stretch-shortening cycle muscles) and maximal voluntary contractions undergo significant changes in women after 3 days of unloading during the follicular phase.
Menstrual cycle effect on strength
This study was planned as a study of the degree of adaptation of the contractile functions of the foot extensor muscles (TS) and the internal architecture of the MG, as one of the three heads of the TS complex that contributes to the generation of MVC, in a 3-day unloading (DI) in a group of healthy young girls with regular menstruation. For the first time, the architecture and biomechanical properties of the muscles of the lower extremities were studied during the performance of vertical jumps in girls under conditions of a 3-day DI. The study showed the reconstruction of the architecture of one of the main locomotor muscles caused by unloading. The main conclusion of this study is that, firstly, the architecture of the MG (lengths and angles of fascicles) changes in response to changes in the position of the ankle joint and the developed force. Secondly, the MG thickness did not change depending on the intensity of muscle contraction, and thirdly, the unloading of the muscular apparatus caused by 3-day unloading under DI conditions was accompanied by an increase in the maximum voluntary torque moment (MVC) and the biomechanical properties of the muscles lower limbs during vertical jump. Contrary to our expectations, the isometric MVC of the foot extensor muscles after 3 days of unloading not only did not decrease, but even increased. It is well known that prolonged mechanical unloading of muscles leads to significant maladjustment of the musculoskeletal system.62
However, in the present study, changes in maximal foot extensor muscle activation appear to be in conflict with the aforementioned disuse paradigms. The main finding confirms our initial hypothesis, as the results of explosive movements (i.e. jumps requiring the stretch-shortening cycle muscles) and maximal voluntary contractions undergo significant changes in women after 3 days of unloading during the follicular phase.
Given the lack of studies using similar performance tests, and girls as subjects under DI conditions, it is extremely difficult to compare the results presented here with other studies. Nonetheless, our results support those of previous studies (that used isometric or isokinetic tests with voluntary contraction) which indicate that different phases of the menstrual cycle do affect muscle strength. The present results suggest that endogenous reproductive hormonal fluctuation characteristic of the MC in women cause significant changes in jumping performance (jump height, flight time) and maximal effort (MVC).
This is the first study to examine the changes in muscle strength plantar flexors, and vertical jumping performants in the later follicular phase of the menstrual cycle in healthy young women. The main finding of this study was that the mechanical properties of muscle were affected by the phase of the menstrual cycle. It is considered that the increased muscle strength and endurance is attributed to high estrogen levels especially, during the follicular phase.20,53,63,64 The post menstrual phase in which there is an increase in estrogen and nor-adrenaline levels is associated with performance improvement65 It has been proposed, that estrogen has a profound effect on the muscle strengt.66 Some investigators have reported an inotropic effect of estrogen on muscle,27 peaking just before ovulation, of a 10% to 11% magnitude,67 and postulated a switching of muscle cross-bridges from low- to high-force generation. Moreover estradiol, in addition to exercise itself, is known to modify growth hormone secretion and metabolism.68 The anabolic effects of this hormone may promote maximal muscle gain and, accordingly, the force of contraction at certain times during al cycle in women.24,69,70
These variations seen in muscle strength may largely be attributed metabolism which are roused due to oscillations in the concentrations of ovarian hormones. The literature intends that the levels of estrogen which is seen peak during follicular phase may promote muscle strength by varying carbohydrate, protein and fat metabolism in the body.71 The levels of estrogen seen during late follicular and early luteal phase stimulates glucose availability and uptake of glucose into type I muscle fibers which act as fuel during short duration exercise.23
In one of the study which found increased muscle strength in terms of time to fatigue at 30% of maximum voluntary contraction between menstrual and follicular phase.69,72 Another study explored the effects of training during follicular phase and the luteal phase on muscle strength, muscle volume and microscopic parameters. There was an increase in muscle strength and muscle diameter in follicular phase the higher concentrations of testosterone and free testosterone.73 Moreover in one of the study conducted it was reported that following menstrual phase in the first 14 days, the female felt improved compared to the subsequent 14 days.16 Sarwar26 and Ronkainen74 reported significantly higher values of maximum voluntary isometric force in quadriceps and handgrip during ovulation compared with the other phases. Phillips27 also showed a higher adductor policies strength during the follicular phase than during the luteal phase. Our findings are consistent with outcomes of previous studies27,28,33,71,75 in which researchers reported of an effect of the menstrual cycle on muscle performance. Petrofsky75 reported greater strength endurance in the late follicular phase compared with that in the luteal phase. A recent systematic review with meta-analysis of the effects of menstrual cycle phase on maximal voluntary force in healthy adult women confirmed that isometric and dynamic strength peaks in the late follicular phase, whereas isokinetic strength peaks at ovulation force. These results indicate that the early follicular phase is unfavorable for muscle force generation in humans. These results indicate that the early follicular phase is unfavorable for muscle force generation in humans.76
The present results study for the isometric strength agreed with these findings. In all these studies, as in the present study, the time of testing was determined only by counting the days in the menstrual cycle. The ideal menstrual cycle is 28 days long, with ovulation at cycle day 14, but the range of a normal cycle length is between 22 and 36 days.77
Ronkainen74 compared 15 postmenopausal monozygotic twin pairs in which one twin had been using hormone replacement therapy for an average of approximately 7 years while the other had no history use. They concluded that ‘long term hormone replacement therapy use was associated with better mobility, greater muscle power and favorable body and muscle. Moreover, Dieli-Conwright78 compared postmenopausal females with or without hormone replacement therapy use reported that those women using hormone replacement therapy had significantly greater upregulation of proanabolic gene expression both at rest and following exercise. Studies using transcranial magnetic stimulation79 indicate that the later follicular phase, when estradiol reaches peak concentration, is coupled with enhanced cortical excitation, in contrast to the early follicular phase that are characterized by cortical inhibition.13 This may be important to emphasize given the clear positive association between excitability and muscle strength.80
Therefore, a logical hypothesis here is that muscle strength should be increased during the late follicular phase due to the increase in excitation and reduced in the early follicular phase due to cortical inhibition. The results obtained in the present study do support this notion as force production was higher after unloading, which comparable to the late follicular phase menstrual cycle. It is considered that the increased muscle strength is attributed to high estrogen levels especially, during the late follicular phase.63 In the present study, there was a remarkable difference noted in muscle strength during the late follicular phase, that reasoned by the higher concentrations of estrogen after unloading
This study hypothesized that biomechanical variables would be reduced at different fall heights following unloading. The results showed that there were differences between drop heights, these differences increased with increasing fall height. However, after unloading the results for all indicators, regardless of jump height, were better.
This is the first study to examine the changes in vertical jumping performants in later-follicular phase of the menstrual cycle in healthy young women. The main finding of this study was that the biomechanical properties of muscle were affected by the phase of the menstrual cycle. It is considered that the increased muscle performance parameter is attributed to high estrogen levels especially, during the follicular phase.20 The post menstrual phase in which there is an increase in estrogen levels is associated with performance improvement.65
In the force-velocity tests (e.g., DJ), the main muscular activity is concentric contractions.81,82 The jump can be considered a ballistic type movement, in which the stretch-shortening cycle (SSC) behavior is very important.83 The mechanical power achieved depends on both neuromuscular coordination and of elastic energy stored in the series elastic components of active muscles and tendons83,84 and especially tendons.85 Both tendons and ligaments are comprised of collagen structural proteins. Estradiol-17b (the main and most active hormone from the group of estrogens) is known to inhibit the collagen synthesis of the ligament86 and consequently increases ligament stiffness. These results suggested that the changes in female steroid hormones during the menstrual cycle did affect the mechanical properties of human muscle and tendon.65
Lower-limb ballistic movements, or SSC stretch-shortening cycle (SSC) muscle actions, have been identified as key determinants of physical performance in women,87,88 and jumping tests are widely used to assess the mechanical capabilities of the lower-limbs and the efficiency of the SSC.88,89 In this study to examine the effects of 3 days DI and late follicular phase of the MC on vertical jumping in young healthy girls. The findings rejects our initial hypothesis, as 'athletic’ performance in these explosive tasks (vertical jumping) requiring SSC muscle actions suffer significant changes after unloading in females over the course of their ovarian MC. It has been proposed, that estrogen has a profound effect on inotropic effect on muscle,27 peaking just before ovulation a 11% magnitude.33 Some investigators have postulated a switching of muscle cross-bridges from low- to high-force generation. Moreover estradiol, to modify hormone secretion and metabolism.90 The anabolic effects of this hormone may promote maximal muscle gain and, accordingly, the force of contraction at certain times during the menstrual cycle in women.70 Estrogen promotes glucose availability and uptake into type I muscle fibers providing the fuel of choice during short duration voluntary movement (MVC) and increases free fatty acid availability and oxidative capacity, favoring endurance performance.25,69
Results of investigations into forces and power output during DJ have shown that these variables may reach larges values after unloading. We assume that an additional factor in performing high jumps may be improved jump technique.91–93 This jumping technique might be a additional factor influencing the effectiveness of executing DJ as testing mode.
The findings suggested that the mechanical energy may absorb the impact with dissipation during the landing. The finding this study suggests that dropping from a higher height may decrease contact time and increase leg, knee, and ankle stiffness. These suggested that subjects may manipulate the knee and ankle joint angles upon contact (and the contact time) to modulate leg, knee, and ankle joint stiffness with different drop heights. This in turn should facilitate the release of more elastic energy during takeoff. Our findings agree with those reported by Farley.94,95 which have suggested that altered body geometry may affect leg stiffness and knee angle upon landing. According to Farley94 altered body geometry may affect leg stiffness and knee angle upon landing. These changes were when the drop jump height was 60 cm. The drop height may elicit changes in the lower extremity geometry before impact to assist in the control of movement. Our findings suggest that the leg and joint stiffness increased may increase after 3 days of unloading in DI conditions in young girls in the late follicular phase of the menstrual cycle. Increased muscle stiffness, and in particular the extensor muscles of the foot, in the follicular phase is confirmed by studies.96,97 This may in turn explain the changes in the mechanical properties of muscles and the muscle-tendon complex during the menstrual cycle. The increased leg stiffness allows for greater storage and release of elastic energy based on SSC35,37 and is associated with an economy of voluntary motion.98
Consequences of unloading on muscle architecture
The present study shows that remodeling of muscle architecture of the main locomotor muscles induced by prolonged (3-day) unloading. Morphological adaptation of skeletal muscles to unloading is the focus of many studies and is achieved via a structural remodeling of contractile machinery. The remodeling of sarcomeres, i.e. the addition or removal of sarcomeres in series and in parallel, is generally believed to be caused by alterations in structures involved in mechano-transduction99 The remodeling is possible to assess at a gross level by evaluating the changes in muscle architecture (that is, fiber length and pennation angle).50,52 The length of muscle fibers strongly affects their contraction velocity91,100 It is of immense importance to deeply understand muscle architecture when interpreting the changes that unloading induces in muscle function because architecture plays a key role in determining the mechanical properties of muscles.44,101 The understanding is additionally important for improving the kinematic efficiency of human movements. A decrease in fiber length and an increase in pennation angle with the increasing muscle length is possible to consider as a factor while explaining muscle tissue “weakness”.102 In this study, a decrease in fiber length upon passive plantar flexion from –15o to +30o suggests that muscle fibers became progressively “weaker” with the increasing ankle joint angle. Ankle angle change assumes change in muscle length.103 It is of interest that the fiber length and pennation angle decreased after a unloading, but the decrease in fiber length was less.
The fiber length was measured at three different joint angles because of the following. If the fiber lengths before and after an unloading (particular DI) are compared at only one particular joint angle and a difference in length is not confirmed, there are chances that the fiber length was initially the same. On the other hand, if the fiber length–joint angle ratio differs between pre- and post-DI measurements, it is impossible to judge about fiber length changes assessed at only one joint angle. In view of this, the fiber length was measured at three different joint angles, including those associated with longer or shorter fibers. Differences in fiber length were observed at all joint angles. It is therefore possible to conclude that the fiber length differed in fact between pre- and post-unloading conditions.
A main finding of the study is that the MVC of the triceps surae complex increased (+10 %) after a 3-day unloading. Changes induced in muscle functions by external factors may be due to changes in contraction processes or changes in nervous (motor) control. In fact, the MVC of a muscle is affected by its force–length relationship, the geometric position of the muscle relative to its joint, and the architectural characteristics of the muscle. Given that the majority of human muscles are pennate, changes in the internal organization of a muscle, which is known as muscle architecture, are important to consider in order to correctly interpreting the functional consequences of unloading.
Muscle architecture, together with internal muscle properties such as fiber composition, affects the functional characteristics of a muscle (e.g., the maximal force and shortening velocity)22,43,104,105 Changes in force generating capacity depend on differences in internal architecture to a greater extent than on differences in fiber composition.22,106 This study is the first attempt to assess changes in the architectural parameters of MG in young girls with a normal menstrual cycle after short-term unloading using ultrasound and to correlate architectural changes with contractile function and joint position.
In the present experiment show that changes after 3-days unloading the fiber length decreased in MG by 2,2 %, and in pennation angle by 8.5 %, respectively. The thickness of MG did not change in response to contraction intensity. Consistency in MG thickness was visualized by comparing resting state with MVC or by comparing different contraction intensities during gradual isometric plantar flexion with the ankle in neutral position. Our findings concerning a constant thickness in the human MG at different muscle lengths and between different contracting stages are in line with in vivo observations of Narici.105 Narici105 using ultrasonography on the same human muscle. Kawakami51,107 reported that of of the MG were reduced by 7 %, respectively after 20 days of bed rest. Interestingly, Reeves108 and Maganaris5 reported only 10 % and 13 % reductions, respectively, in fiber length and pennation angle of the MG muscle after 90 days of bed rest. Moreover, a decrease in pennation angle must make the muscle relatively weaker because, first, parallel sarcomeres are lost. Second, a decrease in fiber length makes sarcomeres function at greater lengths, thus jeopardizing general force production. A greater number of motor units will have to be recruited to maintain the absolute force constant, potentially leading to rapid muscle fatigue.
Fascicle length has been linked to muscle contraction properties.67 Differences in fiber length correspond to differences in the length of sarcomeres, which are located sequentially within the fiber, respectively. Additionally, it is well known that the length of sarcomeres in striated muscles influences the force that can be generated by that muscle fiber.109 Sarcomere lengths can be estimated by dividing the fascicle length by the average number of sarcomeres in series in fascicles.91 Since sarcomere length is a major determinant of muscle force generation potential36 to estimate sarcomere length at maximal force, we divided the fiber length by the number of sarcomeres connected in series in the MG fiber (17,600 Huijing,)110 and superimposed the result on the strength ratio-sarcomere length of human muscles(Figure 5).111 The results indicate that the working range of sarcomere length during voluntary contraction extensor foot is over the upper part ascending of the length-force relationship of both before and after unloading, that a relatively larger force can be generated after unloading.
There was no significant in muscle thickness changes in the present study. However, given the relationship between lengths and angles of fascicles, and muscle thickness, that decrease in muscle thickness can result from a decrease in either or both angles and lengths of fascicles (e.g. small changes in lengths and angles of fascicles may be reflected in large changes in muscle thickness). Although decrease in lengths and angles of fascicle might accompany decrease in muscle size. Nonetheless, other factors must have influenced length of fascicles changes here. The mechanism by which female sex hormones (especially estrogen) could affected architectural changes is unclear. Subjects showed a greater reduction in angles of fascicles and a smaller reduction in lengths of fascicles after unloading in the present study. These changes are likely to result in a similar decrease in muscle thickness.
The mechanism by which female sex hormones (especially estrogen) could influence architectural changes is unclear. One such factor may be fluid (water) retention in the muscles, which may occur after leaving immersion due to dehydration (Figure 6), which will cause an increase in muscle fibers. It is known that during unloading there is an increase in the released fluid and a decrease in the volume of released fluid after leaving unloading.112 Incorporation of water into the muscle would cause an enlargement of the muscle fibers. This would in turn allow then strength changes could have resulted. Thus, architectural changes may have preceded strength gains.
Alternatively, the smaller decrease in fiber length in subjects may have been an adaptation of muscles in response to unloading. A decrease in muscle thickness and fiber angle with a slight decrease in fiber length may mean that in this case part of the developed force of the muscle fiber will be transmitted, since the fibers are oriented at a certain angle (Cos ) relative to the force generation axis. The slight decrease in fiber length partially compensates for the loss of force due to more efficient force transmission to the tendon, since cos of в after unloading increases by 1.3 %. Larger angles of fascicles in muscles aid strength development by allowing more contractile tissue to attach to tendon53. Tendon excursion is greater for a given length of fiber shortening when the angle between the fascicles and the tendon is greater.113 This is because fibers in pennate muscles not only shorten but tremendous rotating during muscle shortening.33,47 Thus, the fibers produce be able to produce greater force depending on their length-tension and force-velocity properties.
Moreover, muscle contraction creates intramuscular pressure, which increases with muscle contraction, and there is a positive relationship between contraction intensity and intramuscular pressure.114 Increased intramuscular pressure is accompanied by curvature of muscle fibers.115 The difference between the pennation values obtained from measurements before and after unloading under isometric conditions with increasing contraction intensity, and the percentage difference between the slope of the angle - contraction intensity curve (Figure 7) indicates a curved shape of the fibers in the muscle.
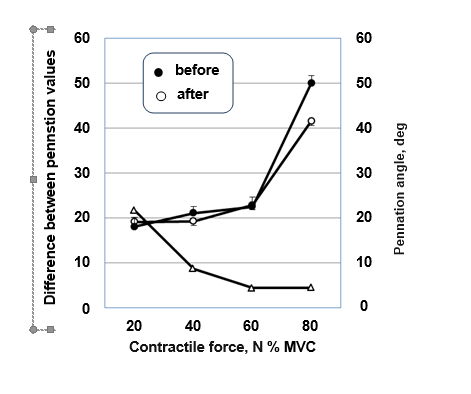
Figure 7 Percentage difference between the angles of fascicles values obtained before and after unloading depending on the intensity of contraction in the neutral ankle position.
Our results conflict with those of Aratow114 and Maganaris5 who determined in vivo changes in pennation angle and fiber length of the MG muscle as a function of ankle joint angle during isometric ankle plantar flexion of subjects under normal gravity conditions. These authors observed similarity between the change in fiber curvature (percentage difference between pennation values) as a function of isometric contraction intensity in MG muscle in the subjects and the change in intramuscular pressure as a function of isometric contraction intensity. In the present study no was found similarity between the changes in fiber curvature (percentage difference between slope values) depending on the intensity of isometric muscle contraction in the subjects and the change in intramuscular pressure depending on the intensity of isometric contraction. The study noted a decrease in intramuscular pressure with increasing contraction intensity. As you know, fluid creates pressure and since unloading is accompanied by loss of fluid, intramuscular pressure decreases accordingly. The latter causes less bending of the fibers, which ensures effective transfer of the developed force to the tendon.
To our knowledge, our study is the first to demonstrate a linear relationship between muscle architecture variables (Figure 8). In the studied MG, a high correlation was observed between the PCSA index and the pennation angle of the fibers ( = 0.61, p < 0.01) after unloading. Although the relationship between the PCSA index and the fiber pennation angle before unloading had a relatively weak relationship ( = 0.29, p < 0.05). MG pennation angles increased to a greater extent with increasing PCSA index before unloading, while a different picture was observed after unloading, with increasing pennation angles PCSA index to a lesser extent increased. We believe that the increase in connection after unloading in our study is associated with an important role in the production of sex hormones in the late follicular phase of the menstrual cycle, which changes the mechanical properties of the muscle tendon and musculotendinous complex. Thus, changes in muscle architecture can explain, at least partially, the differences in functional properties observed in our study.
The role of neural activation in the control of voluntary movement cannot be excluded. Two studies postulated that the main mechanism responsible for the increase in neural activation following unloading may be increased spinal excitability82,116 and point of view was confirmed experimentally.117 Lundbye-Jensen and Nielsen117 demonstrated that after unloading the increase reflex excitability (increased H-reflex amplitude) is caused by both presynaptic inhibition and homosynaptic postactivation depression. Since the main contribution to the work performed is made by the triceps surae muscle during locomotion,118 an increase in neural activity may likely be an additional factor in the increase in muscle functions.
The muscle contractions (MVC and vertical jumping requiring SSC muscle actions) the menstrual cycle and showed the greatest performance, that is were significantly higher and forceful during the late-follicular phase. The changes seen during normal menstrual cycle can affect the recorded functions of the performed voluntary movements and therefore they must be taken into account during the preparation and selection of the experiment program.
This study demonstrates increase in muscle strength and muscle endurance between different determined menstrual cycle phases in young women in unloading. In contrast to other studies when only men participate in the study, a decrease in strength and endurance is detected during unloading. However, since this is the first study in young girls, further research is needed to elucidate the interactions and consequences of the unloading and functional effects associated with hormonal changes during the menstrual cycle
The limitations of this study are as follows. This is a study was performed without measurement the concentration of sex hormones,62 which would have been much more relevant in classifying different phases of menstrual cycle. The phases of the menstrual cycle were assessed from the first day of bleeding and by noninvasively measuring basal body temperature.
The menstrual cycle affects the mental and physical conditions of young female, and female hormones influence the skeletal muscle metabolism. We postulated that changes in female hormones induced by the menstrual cycle affect the outcomes of biomechanical properties during drop jumps and voluntary contractions. Therefore, it is obvious that the female menstrual cycle is a key element in the change of functions, and these should be taken into account in the preparation of studies on the unloading of the muscular apparatus.
The overall results of this study have important implications for astronaut/cosmonaut coaches from practical view, it will be very important to consider menstrual phase when programming training sessions for female astronaut/cosmonaut by decreasing the training load during the pre-menstrual phase to limit neuromuscular fatigue accumulation. Additionally, the follicular phase appears to be the best time for high-intensity training to improve performance. Productivity, especially during periods of critical operator activity. This indicates that the frequency, volume and intensity of training can be selected individually based on the biological potential of the menstrual phase. Future investigations should focus on the concept of training programs tailored to different phases of the menstrual cycle.
The author would like to express my appreciation and sincere gratitude to our participants for their commitment and willingness to share with us data about their menstrual cycle and hormonal contraceptives, the medical and engineering staff for their attention to detail and hard work during the study, doctors of the clinical department of the Institute of Biomedical Problems of the Russian Academy of Sciences (head of the department E. Sigaleva) for participation in the selection of volunteers, I. Ponomarev for technical assistance during data collectionб as well as expresses special gratitude to G. Vasilyeva for collecting water balance data, preliminary analysis and kindly providing water balance material, and also to E. Tomilovskaya for organizing this experiment. The author deep expresses gratitude for technical assistance provided to Albartas Skurvydas, Jorn Dainauskas and Aivaras Ratkevicius (Lithuanian Sports University, Kaunas, Lithuania).
This study was supported by grants from the Russian Science Foundation (number 19-15-00435).
The author declares that there are no conflicts of interest.

©2024 Koryak. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.