eISSN: 2473-0815


Research Article Volume 12 Issue 2
State institution “Republican Scientific and Practical Center of Mental Health”, Belarus
Correspondence: Kuchinskaya Аliaksandra, Research Associate, State institution “Republican Scientific and Practical Center of Mental Health”, Minsk, Belarus, , Tel +375297531416
Received: July 27, 2024 | Published: August 15, 2024
Citation: Kuchinskaya A, Mosse К, Dakukina T, et al. Genetic factors in the formation of sexual identity and the emergence of gender dysphoria. Endocrinol Metab Int J. 2024;12(2):62-66. DOI: 10.15406/emij.2024.12.00349
Relevance: The modern theory of the occurrence of gender disorders considers differences in sexual behavior as a result of the action of genes that are involved in the biosynthesis and functioning of sex hormones. Research on the differences between the transgender cohort and the general population may be useful in addressing this issue.
Research materials and methods: The following genes and polymorphic variants were selected for genetic testing based on literature data: trinucleotide CAG repeats in the AR gene; dinucleotide TA repeats – 1174(TA)n in the ESR1 gene; dinucleotide TA repeats in the SRD5a2 gene; polymorphism G472A (rs4680) in the COMT gene. Testing was also performed for the presence of the SRY gene and sections of the Y chromosome including the AZF loci. For the molecular genetic analysis of the selected polymorphic variants, testing protocols have been developed using fragment analysis and real-time PCR methods.
Results: Molecular genetic testing of the SRY gene and microsatellite markers of the Y chromosome showed their correspondence to the patient's gender in all samples. Differences between the study group and the control group in the frequency of occurrence of CAG repeats of the AR gene, alleles of polymorphic sequences in the ERa, SRD5A2, and COMT genes were revealed.
Conclusion: The results obtained suggest that gender dysphoria has a polygenic basis, including both the influence of individual polymorphisms and interactions between several genes that can alter the sexual differentiation of the brain, contributing to the development of gender dysphoria.
Keywords: gender dysphoria, genetic testing, genes SRY, AR, ESR1, SRD5a2, COMT
The modern theory of the occurrence of gender disorders considers differences in sexual behavior as a result of the action of genes that are involved in the biosynthesis and functioning of sex hormones. If we assume that the central role in the development of the brain in terms of sex differences belongs to sex hormones, then changes in the level of sex hormones are responsible for gender dysphoria.1–4
To establish this fact (or refute it), studies have been initiated to detect differences between the transgender cohort and the general population. There have also been suggestions about the influence of individual polymorphisms and the interaction between several genes that can alter the sexual differentiation of the brain. For example, the role of the anti-aging gene Sirtuin 1 has been studied, which may be crucial for the interaction between several genes that can influence sexual differentiation in the brain. Sirtuin 1 is important to effects of estrogen and testosterone with relevance to gender dysphoria. The role of Sirtuin 1 inhibitors versus Sirtuin 1 activators may determine sexual differentiation of the brain and the development of sexual dysphoria.5–8
Currently, attempts are being made to detect and confirm the significance of candidate genes involved in the formation of sexual identity and, accordingly, in the occurrence of gender dysphoria.9,10
The key role in male sex determination belongs to the SRY (sex determining region Y) gene. The SRY gene is located in close proximity to the region of homologous pairing of X and Y chromosomes. During the process of meiotic exchange between the X and Y chromosomes, the section of the Y chromosome containing the SRY gene can be translocated to the X chromosome, which can lead to the formation of gametes with the Y chromosome that has lost the SRY gene and the X chromosome carrying this gene. When fertilized with such egg gametes, it is possible to give birth to men with karyotype XX with translocation of the SRY gene or women with karyotype XY, but with deletion of the SRY gene.11
Patients with complete X chromosome monosomy may be carriers of latent mosaicism, including a clone of cells containing the Y chromosome. About 80% of patients with X-inversion of sex have a small part of the Y chromosome in the genome, which is invisible under light microscopy. Sections of the Y chromosome in such patients are translocated, as a rule, to the X chromosome received from the father. Only a small portion of the Y chromosome containing the SRY gene can be translocated, since the presence of the SRY gene in the genome, and not the entire Y chromosome, is sufficient for the full development of the male phenotype.12,13
Studies of the association of candidate genes are based on determining the relationship of functional variants in genes that transmit sex hormone signals with gender dysphoria. It is suggested that functional variants may alter the transmission of sex hormone signals, causing atypical sexual differentiation of the developing brain in those who will later experience gender dysphoria. Some associations have been identified in the study of polymorphic variants in the genes AR, ERa, SRD5a2, COMT.14–20
Most of the studies were limited to small sample sizes. The androgen receptor (AR) gene is localized on the X chromosome (Xq11). The gene consists of 8 exons, and their structural organization is similar to the genes of other steroid hormone receptors. One of the factors influencing the function of the AR gene is a polymorphic locus in exon 1 of the gene containing a different number (from 5 to 70) of trinucleotide CAG repeats. This site encodes a polyglutamine tract in the AR protein, the length of which in healthy individuals, as a rule, does not exceed 40 repeats.21
Polyglutamine sequence polymorphism is widespread in the population, however, it has its own functional significance: the length of the polyglutamine section of the AR encoded by CAG repeats can affect the activity of the receptor. The number of CAG repeats is associated with some androgen-dependent disorders, such as polycystic ovary syndrome,22 male infertility, prostate cancer,23 and may even have an effect on growth in boys.24
High insensitivity to androgens in brain tissues may contribute to the development of gender dysphoria, and therefore further study of the effect of different variants of the number of repeats (CAG)n in the transsexual population is necessary. AR is an attractive candidate for research, because sometimes it is possible to observe a complete loss of its function in individuals with a female phenotype and female sexual identity with genotype 46HU.25,26
In addition, polymorphism (SAP) of t repeats is known to have a regulatory effect. In a 2005 study, it was revealed that the long sequence (SAP) of the t repeat is very common in transsexual MeA. Later, the same discovery was confirmed in a 2009 study on 112 transsexuals of the Caucasian race. But exactly the same case-control study in the Asian population did not confirm this phenomenon.27
Estrogen receptors (ER) are represented by two subtypes similar in structure and a ligand binding domain. ER of both subtypes are widespread throughout the human body, each in different tissues and with a different expression pattern. ERα mRNA is synthesized in large quantities in the hypothalamus and amygdala of the brain. ERα plays an extremely important role in the regulation of reproductive neuroendocrine behavior (and the formation of adequate fertility).
The ESR1 gene encoding the ERα protein is located on chromosome 6 (6q25.1). Its transcription is regulated by promoters, which give rise to mRNA variants with 5`-untranslated regions. Polymorphism of repeats – 1174(TA) n in ESR1 is associated with conditions such as coronary heart disease, decreased bone mineral density, familial premature ovarian failure, and endometriosis. The functional importance of repeats of these nucleotide sites is still not fully clear, although there are reports of an important role of these repeats in the functioning of untranslated regions and their effect on gene expression. In addition, polymorphism is associated with irritability, neuroticism, severe mental disorders, antisocial behavior, and conductive disorders.
ESR1, encoding the ERα protein, is located on chromosome 6 (6q25.1). Its transcription is regulated by promoters, which give rise to mRNA variants with 5`-untranslated regions. Polymorphism of repeats – 1174(TA) n in ESR1 is associated with conditions such as coronary heart disease, decreased bone mineral density, familial premature ovarian failure, and endometriosis. The functional importance of repeats of these nucleotide sites is still not fully clear, although there are reports of an important role of these repeats in the functioning of untranslated regions and their effect on gene expression. In addition, polymorphism is associated with irritability, neuroticism, severe mental disorders, antisocial behavior, and conductive disorders.
The SRD5α2 gene, encoding 254 amino acids of the 5-α-reductase enzyme, is located on chromosome 2p23, contains 5 exons and 4 introns. Mutations in this gene lead to hypovirilization of individuals with genotype 46 XY. There are some variants of polymorphism of this gene that are associated with polycystic ovarian syndrome. One of them, identified in the 3` untranslated region, consists of a variable number of dinucleotide repeats of TA. Three clusters of base pair repeats were identified, which were assigned the value (TA)0, including 96% of alleles, and variants of alleles (TA)9 and (TA)18. Although this polymorphism does not affect the structure of the resulting protein, it can affect the stability of mRNA and thus alter enzymatic activity.
An increase in the incidence of hypospadias with overexpression of the SRD5α2 gene was found in comparison with the control group. Polymorphism of this gene may be associated with hyper virilization, which may be the cause of gender dysphoria.
The COMT gene encodes an enzyme involved in the metabolism of neuropeptides (oxytocin, vasopressin, etc.), including dopamine catabolism in the intercellular space, due to a lack of a dopamine transporter in the prefrontal cortex. The physiological role of the enzyme is to eliminate catecholamines from the synaptic cleft by a methylation reaction. The dynamics of these neuropeptides in different parts of the brain regulates social behavior. In turn, the activity of catechol-O-methyltransferase is influenced by a number of factors, including the genetic polymorphism of the enzyme.
The most well-known polymorphism is G472A (rs4680), which is a single nucleotide substitution in exon 4 of the gene with the substitution of the amino acid Val for Met in codon 158 for the isoform MV-COMT and in codon 108 for S-COMT. The widespread functional polymorphism of Val158Met plays an essential role in the metabolic activity of the enzyme, affects the degradation of dopamine in the prefrontal cortex and affects the cognitive properties of an individual (working memory, executive functions). The Met allele is associated with low dopaminergic activity and thermal stability, which is accompanied by an increase in dopamine levels in the prefrontal cortex, and the Val allele is associated with high dopaminergic activity (3-4 times higher than that of carriers of the Met allele). The carriage of the Val allele increases the activity of catechol-O-methyltransferase by 40%, causes a low level of dopamin515e in the synaptic cleft of the prefrontal cortex, and affects dopamine activity in the anterior brain.
Men are characterized by higher activity of the enzyme COMT compared to women. The nature of associations of genetic markers at the COM locus with psychiatric phenotypes, including schizophrenia, also reveals sexual specificity. The described sex differences are explained by the depressing effect of estrogens on the activity of catechol-O-methyltransferase.
The following genes and polymorphic variants were selected for genetic testing based on literature data:
All patients were also tested for the presence of the SRY gene and Y chromosome regions including AZF loci. For the molecular genetic analysis of the selected polymorphic variants, testing protocols have been developed using fragment analysis and real-time PCR methods.
DNA isolation from the buccal epithelium was carried out with a set of "Nucleosorb Package A" (ODO “Primtech”). Lysing solution "A" (400 mcl) was introduced into tubes with rods (buccal epithelium) using tips with an aerosol barrier and incubated in a thermostat (at rpm 1080) for 30 minutes at 64°C. After the end of incubation, a universal sorbent of 20 µl was added. The contents were mixed and incubated in a thermostat for 5 minutes at 64°C.
Then the contents were mixed on a vortex and left at room temperature for 2 minutes. After that, the sorbent was deposited in test tubes by centrifugation at 10000 rpm for 30 seconds. Without capturing the sorbent, the filler liquid was removed.
The sorbent was dried in a thermostat for 5-10 minutes at 64 ° C, while the tube lids should be open. A TE buffer of 35 µl was added to the tubes for DNA elution. Mixing was performed on the vortex until the sorbent was completely resuspended and placed in a thermostat for 5 minutes at 64 ° C, with periodic shaking on the vortex. The tubes were then centrifuged at 12000 rpm for 1 minute and the filler liquid containing DNA was transferred to clean tubes.
Analysis of the SRY gene and microsatellite markers of the Y chromosome. For the study, a multiplex polymerase chain reaction (PCR) system with 8 pairs of primers was used to investigate the presence of the SRY gene and Y-specific markers: sY84 and s Y86 (AZFa), sY127 and sY134 (AZFb), sY254 and s Y255 (AZFc). The ZFY/X gene (the "zinc finger" gene present on both the Y and X chromosomes) was used as an internal control.
The reaction mixture with a final volume of 25 µl contained 1 x PCR buffer, 3.5mM MgCl2, 200 µm dNTP, 3 ppM primers and 1.5 units of Taq polymerase. After denaturation of the samples at 95 ° C, 30 amplification cycles followed for 8 minutes under the following temperature and time conditions: 30 seconds of denaturation at 95°C; 60 seconds of annealing at 57 ° C and 60 seconds of synthesis at 72°C. At the final stage of synthesis, the tubes were kept for 8 minutes at 72 ° C. PCR products were analyzed using automatic capillary electrophoresis in a SeqStudio genetic analyzer. Data processing and marker detection were performed using the Gene Mapper 4.1 computer software package.
Analysis of microsatellite polymorphism in the AP, ESR1 and SRD5a2 genes. Microsatellite polymorphism was studied by direct determination of the number of di- and trinucleotide repeats using PCR and capillary electrophoresis. The optimal composition of the reaction mixture and temperature-time conditions of PCR were selected for each gene. Amplification was performed with primers flanking DNA regions containing polymorphic repeats (Table 1).
|
Gen |
Primers |
Annealing temperature (°C) |
the size of the PCR product (bp) |
|
AР |
F 5’ FAM - GTGCGCGAAGTGATCCAGA 3’R 5’ GTTTCCTCATCCAGGACCAGGTA 3’ |
55 |
211-289 |
|
ERα |
F 5’ FAM -GACGCATGATATACTTCACC 3’R 5’ GCAGAATCAAATATCCAGATG 3’ |
56 |
154-196 |
|
SRD5A2 |
F 5’ FAM -CTGATGAAAACTGTCAAGCTG 3’R 5’ GCCAGCTGGCAGAACGCCAGGAGAC 3’ |
56 |
89-108 |
Table 1 Primers used to determine di- and trinucleotide repeats in the AP, ESR1 and SRD5a2 genes
PCR products were analyzed using automatic capillary electrophoresis in a SeqStudio genetic analyzer. Data processing and marker identification were performed using a computer software package Gene Mapper 4.1.
Determination of Val158/Met (rs4680) polymorphism of the catechol–O–methyltransferase (COMT) gene
Polymorphism determination (rs4680) was performed by real-time PCR. The reaction mixture with a final volume of 10 µl contains 5 µl of 2×PCR buffer and 0.5 µl of primers with TaqMan® probes. To the test tubes for PCR 9 µl of the amplification mixture and 1 µl (20 ng) of the DNA sample are added.
Place the test tubes in an amplifier and denature the samples at 95°C for 10 minutes. After denaturation, 40 amplification cycles follow under the following temperature and time conditions: 15 sec. denaturation at 95°C; 60 sec. annealing and synthesis at 60°C.
The results of molecular genetic testing of the SRY gene and microsatellite markers of the Y chromosome showed their correspondence to the patient's gender in all samples. All men were found to have the SRY gene and no Y chromosome deletions. In all DNA samples from women, only a fragment corresponding to the ZFY/X control gene was found.
The study of trinucleotide repeats of the AR gene in a group of transgender men revealed 8 different allelic variants in the range from 19 to 28 CAG repeats with the most frequent allele (27.7%) having 23 repeats. The study of trinucleotide repeats of the AR gene in a group of transgender men revealed 8 different allelic variants in the range from 19 to 28 CAG repeats with the most frequent allele (27.7%) having 23 repeats.
In the group of women, 13 different allelic variants were observed in the range from 13 to 32 CAG repeats with the most frequent allele (23.4%) having 21 repeats. According to the number of CAG repeats, in accordance with the generally accepted comparison practice, all the examined were divided into subgroups having short ((CAG) n ≤18), medium ((CAG) n 19-25) and long ((CAG)n ≥26) variants of the polyglutamine tract. Alleles with the number of CAG repeats from 19 to 25 prevailed in both groups: men (69.2%) and women (81.2%) (Table 2). As for short and long repetitions, the opposite pattern was observed in the groups. In men, there are no short repeats, while alleles of more than 26 repeats account for 30.8%. Among women, the frequency of short and long repetitions is the same - 9.4%.
|
Quantity of GAG repeats (Min Max) |
Median (Me) |
Occurrence of GAG alleles of the AR gene, n (%) |
|||
|
Short |
Middle |
Long |
|||
|
(CAG) n ≤18 |
(CAG) n = 19–25 |
(CAG) n ≥26 |
|||
|
Women (32) |
13-32 |
21.7 |
6 (9.4) |
52 (81.2) |
6 (9.4) |
|
Men (16) |
19-28 |
23,4 |
- |
11 (69,2) |
5(30,8) |
|
Control Men % |
18-31 |
22 |
2,3 |
81,7 |
16 |
Table 2 Frequency of occurrence of CAG repeats of the AR gene in the studied groups. Occurrence GAG-alleles
The number of CAG repeats of less than 18 is clinically associated with an increased risk of prostate cancer, an earlier age of manifestation of the disease. This may most likely be due to androgen receptor overexpression. A large number of CAG repeats are associated with androgen receptor insufficiency, which may lead to a loss of tissue sensitivity to androgens and, in turn, affect the process of spermatogenesis. In patients with long polyglutamine alleys, there was also a tendency to defects in spermatogenesis. (Table 2)
Our results indicate that the inhibitory effect of CAG repeat length on androgen levels in men may be one of the causes of gender dysphoria. Associations with gender dysphoria were revealed by studying the allele frequencies of polymorphic sequences in the genes ERa, SRD5A2, and COMT. It can be assumed that these genetic variants are functional, which will allow in the future to study their effect on the level of sex hormones responsible for gender dysphoria.
In the ERa gene, for example, short TA repeats overrepresented in transgender women are also associated with low bone mineral density in women as a result of decreased estrogen signaling, according to the literature. The distribution of alleles of this polymorphism was equally bimodal in both groups with two peaks of 13 and 22 repeats (Figure 1). The number of repeats ranged from 12 to 24. The number of women who are carriers of two short (12-14) TA repetitions (S/S) is 31.2%, whereas among men this indicator is 18.7% (Table 3) (Figure 1).
|
Gen |
The allelic variant |
Patients with gender dysphoria |
Control group (%) |
|
|
Women (32) |
Мen (19) |
|||
|
SRD5α2 |
TA(0) |
62 (96,9) |
28 (82,5) |
91,5 |
|
3`untranslated region (UTR) TA repeat |
TA(9) |
2 (3,1) |
6 (17,5) |
8,5 |
|
Era |
S/S |
10 (31.2) |
3 (18.7) |
27 |
|
promoter |
S/L |
14 (43.8) |
9 (56.3) |
50 |
|
region TA repeat |
L/L |
8 (25) |
4 (25) |
23 |
|
COMT |
G/G |
6 (18,7) |
6 (31,6) |
29,4 |
|
rs4680 |
G/A |
24 (75) |
4 (21,0) |
45 |
|
A/A |
2 (6,3) |
9 (47,4) |
25,6 |
|
Table 3 Allele frequencies of di- and trinucleotide repeat polymorphism in the AP, ESR1 and SRD5a2 genes of patients with gender dysphoria and in the control group
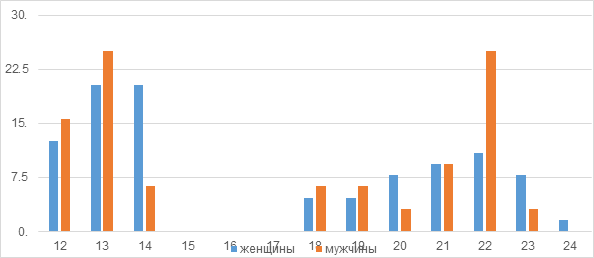
Figure 1 Frequencies of TA repeat polymorphism alleles in the ER2 gene in transgender women and men.
Even more significant differences were found in the distribution of alleles of the nucleotide polymorphism of the SRD5A2 gene. The frequency of the T A9 allele in the group of men was 17.5% and only 3.1% in women. These results confirm the previously proposed hypothesis that the T A9 allele is associated with reduced levels of dihydrotestosterone and the level of this potent androgen may contribute to the occurrence of gender dysphoria in men.
Significant differences were also found in the distribution of alleles of the G472A (rs4680) polymorphism in the COMT gene. In the group of men, the frequency of homozygous carriage of the A/A allele was 47.4%, and in the group of women only 6.3%.
The COMT gene encodes the enzyme catechol-O-methyltransferase, which ensures the conversion of catechol estrogens into less active metabolites.25 Polymorphism of r4680 leads to the replacement of the amino acid valine with methionine in codon 158, differentiating the alleles COMT G/G (high activity) and COMT A/A (low activity), which leads to a 3-4-fold decrease in enzyme activity. Thus, homozygous carriers of the A allele have a distinctly reduced enzyme activity, which leads to less effective inactivation of catechol estrogens. It has previously been shown that middle-aged men with the COM A/A genotype have higher serum estradiol levels than men with other genotypes.
Thus, the current results of the study of polymorphic variants of genes involved in the biosynthesis and functioning of sex hormones confirm the hypothesis that gender dysphoria has a polygenic basis, including both the influence of individual polymorphisms and interactions between several genes that can alter the sexual differentiation of the brain, contributing to the development of gender dysphoria in transgender people.
None.
The authors declare that there are no conflicts of interest.

©2024 Kuchinskaya, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.