eISSN: 2473-0815


Review Article Volume 12 Issue 3
1School of Biotechnology, Gautam Buddha University, India
2School of Physical Science, Jawaharlal Nehru University, India
Correspondence: Dr. Imteyaz Qamar, Assistant Professor School of Biotechnology, Gautam Buddha University, Greater Noida, U.P, 201312, India
Received: February 23, 2024 | Published: August 27, 2024
Citation: Banerjee P, Urmi R, Sharma N, et al. Exploring the role of pyridoxal kinase: a key player in vitamin B6 metabolism. Endocrinol Metab Int J. 2024;12(3):68-75. DOI: 10.15406/emij.2024.12.00350
Cancer remains one of the most challenging diseases to treat, demanding innovative approaches to combat its complexity and heterogeneity. In recent years, Pyridoxal kinase (PDXK), a critical enzyme in the vitamin B6 metabolic pathway, has emerged as a promising target in the pursuit of effective cancer therapies. PDXK, responsible for phosphorylating vitamin B6 to its active forms, plays a pivotal role in various cellular processes, including DNA synthesis, amino acid metabolism, and immune regulation. Dysregulation of PDXK expression has been implicated in cancer, contributing to tumorigenesis and progression. Recent advances in small molecule inhibitors and activators targeting PDXK have showcased their potential to alter cancer cell behavior. These molecules hold promise not only as standalone treatments but also as adjuvants to conventional therapies, augmenting their efficacy. Moreover, PDXK modulation has a profound impact on tumor metabolism. By perturbing vitamin B6 homeostasis, it disrupts the bioenergetics and redox balance within cancer cells, rendering them vulnerable to therapeutic intervention. Combining PDXK modulation with existing cancer therapies, such as chemotherapy or immunotherapy, offers the tantalizing prospect of synergistic treatment approaches, potentially enhancing therapeutic outcomes while minimizing side effects. This review explores the therapeutic potential of PDXK modulation as a novel strategy in the battle against cancer.
Keywords: pyridoxal kinase, pyridoxal 5'-phosphate, cancer therapy, vitamin b6 metabolism
Non communicable diseases (NCDs) have become the primary contributors to global mortality, with cancer projected to take the forefront as the leading cause of death and a significant obstacle to extending life expectancy across all nations in the 21st century.1 According to World Health Organization (WHO) data from 2015, Cancer serves as the leading or secondary factor contributing to mortality in individuals below the age of 70 in 91 out of 172 countries. Additionally, it occupies the third or fourth position in an additional 22 countries in terms of causes of death. In 2018, as per the GLOBOCAN study, the deadliest cancers were lung, stomach, liver, breast, colorectal, and blood cancers like leukemia, causing a significant number of fatalities worldwide.2
Among the various forms of leukemia, Acute Myeloid Leukemia (AML) stands as the most prevalent type of acute leukemia. Despite its rarity, constituting merely 1% of all cancer cases, AML carries the unfortunate distinction of being the most lethal form of leukemia. The five-year survival rate for individuals diagnosed with AML, indicating the percentage of people expected to survive five years after diagnosis, currently stands at 29.5%.3,4
The primary hallmark of acute myeloid leukemia (AML) is the abnormal proliferation and invasion of undifferentiated blasts of the bone marrow (BM), lymph nodes and spleen.5 Recent studies suggest that the genetic drivers of cancer development (mutated oncogenes, tumor suppressors, etc.) can hijack cellular metabolism to keep cancer cells alive and growing. Metabolic reprogramming plays a vital role in cancer development, and some cancer-causing mutations directly impact metabolic pathways. Examples include isocitrate dehydrogenase (IDH) missense mutations in leukemia, glioma, sarcoma, and cholangiocarcinoma, succinate dehydrogenase (SDH) loss-of-function mutations in pheochromocytoma and paraganglioma, and FH hereditary mutations in renal cell cancer.
It is believed that vitamins are essential nutrients which are needed in limited amounts to support the health of the organism, including a number of metabolic processes related to cancer. Vitamins C and D, for instance, have recently been implicated in controlling cancer-related processes, and perturbations can influence cancer phenotypes.6,7 Chen and colleagues demonstrated that the enzyme pyridoxal kinase (PDXK) facilitates the phosphorylation of vitamin B6, generating the active form known as pyridoxal 5'-phosphate (PLP). This active form, in turn, plays a crucial role in regulating two essential metabolic enzymes necessary for the growth of acute myeloid leukemia (AML) cells.8
Healthy cells keep their use of vitamin B6 under control. Leukemic cells exhibit an intense dependence on vitamin B6. The distinction between responsible vitamin B6 utilization and addiction in these cells often hinges on a singular gene—the one responsible for expressing the PDXK enzyme. If drugs capable of inhibiting or disrupting this enzyme or its counterparts within the vitamin B6 pathway could be developed, novel treatment approaches could be explored for combating acute myeloid leukemia.9
All living organisms consist of diverse cell types that execute a range of metabolic functions crucial for survival. These metabolic processes rely on specialized enzymes, predominantly composed of proteins, to facilitate various biochemical reactions. These enzymes are produced through the translation of specific genes encoding them. One notable example is Pyridoxal Kinase, a crucial human enzyme encoded by the PDXK gene.10 Pyridoxal Kinase plays a pivotal role in catalyzing the phosphorylation of Pyridoxine (commonly known as Vitamin B6), resulting in the formation of Pyridoxine 5’-phosphate.11
PDXK gene is located on the 10th chromosome of Mus musculus (House mouse). Pyridoxal kinase enzyme is also present in humans and is transcribed from PDXK gene which is present on chromosome number 21; open reading frame 124 (NCBI Gene, 2023). The enzyme, named for its physiological role in phosphorylating pyridoxine, belongs to the transferases family, utilizing the alcohol group as an acceptor. It plays a crucial role in synthesizing pyridoxal 5’-phosphate (PLP), pyridoxine 5’-phosphate (PNP), and pyridoxamine 5’-phosphate (PMP) from the dietary vitamin B6 vitamers pyridoxal (PL), pyridoxine (PN), and pyridoxamine (PM). PLP serves as the active coenzyme form of vitamin B6, essential as a cofactor in over 140 enzymatic reactions.
These reactions encompass the biosynthesis and catabolism of amino acids and nucleic acids, as well as the regulation of glucose, sphingolipid, and fatty acid metabolism.12,13 The enzyme is primarily located in the cytoplasm and operates as a homodimer. Furthermore, various alternatively spliced variants with biological significance have been identified.14 Lainé-Cessac P, Cailleux A, Allain P, elucidated the mechanisms underlying the inhibition of pyridoxal kinase by several drugs.14 The maximum expression of PDXK gene can be observed in the ovaries and kidneys of adult mice while the minimum expression of it is in liver and testis of adult mice.15
In 2004, Martin K. Safo and colleagues published a report unveiling the crystal structure of PdxY, a protein in Escherichia coli with homology to PDXK.16 simultaneously, researchers explored the three-dimensional structures of sheep brain PDXK both independently and in conjunction with various ligands. These ligands included PDXK/ATP, PDXK/AMP-PCP/pyridoxamine, PDXK/ADP/PLP, and PDXK/ADP. These investigations have significantly advanced our understanding of PDXK's catalytic mechanism.17,18
The catalytic process of PL kinase involves the addition of the γ-phosphate from ATP to the 5’ alcohol of PN, PM, and PL, resulting in the formation of PNP, PMP, and PLP, respectively (Figure 1).19
The Protein Data Bank currently includes amino acid sequences for over 100 close homologs of PL kinases from both prokaryotic and eukaryotic organisms, exhibiting sequence identities ranging from 24% to 90% when compared to the human enzyme. Comprehensive sequence analyses of these PL kinase enzymes have been documented.20 Several of these enzymes have undergone detailed investigations aimed at exploring their substrate-binding sites, employing inhibitors, chemical modification techniques, spectroscopy, and X-ray crystallography.21,22
Purification of PL kinases has been achieved from bacterial, plant, and mammalian sources. Evidence indicates that most eukaryotic organisms possess a single PL kinase, encoded by a pdxK gene. In contrast, prokaryotes harbor two isoform PL kinases, encoded by pdxK and pdxY genes. Notably, the enzyme coded by the pdxY gene lacks catalytic activity, and subsequent references to PL kinase typically pertain to the enzyme coded by the pdxK gene.23,24
All living organisms consist of diverse cell types that execute a range of metabolic functions crucial for survival. These metabolic processes rely on specialized enzymes, predominantly composed of proteins, to facilitate various biochemical reactions. These enzymes are produced through the translation of specific genes encoding them. One notable example is Pyridoxal Kinase, a crucial human enzyme encoded by the PDXK gene.10 Pyridoxal Kinase plays a pivotal role in catalyzing the phosphorylation of Pyridoxine (commonly known as Vitamin B6), resulting in the formation of Pyridoxine 5’-phosphate.11
PDXK gene is located on the 10th chromosome of Mus musculus (House mouse). Pyridoxal kinase enzyme is also present in humans and is transcribed from PDXK gene which is present on chromosome number 21; open reading frame 124 (NCBI Gene, 2023). The enzyme, named for its physiological role in phosphorylating pyridoxine, belongs to the transferases family, utilizing the alcohol group as an acceptor. It plays a crucial role in synthesizing pyridoxal 5’-phosphate (PLP), pyridoxine 5’-phosphate (PNP), and pyridoxamine 5’-phosphate (PMP) from the dietary vitamin B6 vitamers pyridoxal (PL), pyridoxine (PN), and pyridoxamine (PM). PLP serves as the active coenzyme form of vitamin B6, essential as a cofactor in over 140 enzymatic reactions.
These reactions encompass the biosynthesis and catabolism of amino acids and nucleic acids, as well as the regulation of glucose, sphingolipid, and fatty acid metabolism.12,13 The enzyme is primarily located in the cytoplasm and operates as a homodimer. Furthermore, various alternatively spliced variants with biological significance have been identified.14 Lainé-Cessac P, Cailleux A, Allain P, elucidated the mechanisms underlying the inhibition of pyridoxal kinase by several drugs.14 The maximum expression of PDXK gene can be observed in the ovaries and kidneys of adult mice while the minimum expression of it is in liver and testis of adult mice.15
In 2004, Martin K. Safo and colleagues published a report unveiling the crystal structure of PdxY, a protein in Escherichia coli with homology to PDXK.16 simultaneously, researchers explored the three-dimensional structures of sheep brain PDXK both independently and in conjunction with various ligands. These ligands included PDXK/ATP, PDXK/AMP-PCP/pyridoxamine, PDXK/ADP/PLP, and PDXK/ADP. These investigations have significantly advanced our understanding of PDXK's catalytic mechanism.17,18
The catalytic process of PL kinase involves the addition of the γ-phosphate from ATP to the 5’ alcohol of PN, PM, and PL, resulting in the formation of PNP, PMP, and PLP, respectively (Figure 1).19
The Protein Data Bank currently includes amino acid sequences for over 100 close homologs of PL kinases from both prokaryotic and eukaryotic organisms, exhibiting sequence identities ranging from 24% to 90% when compared to the human enzyme. Comprehensive sequence analyses of these PL kinase enzymes have been documented.20 Several of these enzymes have undergone detailed investigations aimed at exploring their substrate-binding sites, employing inhibitors, chemical modification techniques, spectroscopy, and X-ray crystallography.21,22
Purification of PL kinases has been achieved from bacterial, plant, and mammalian sources. Evidence indicates that most eukaryotic organisms possess a single PL kinase, encoded by a pdxK gene. In contrast, prokaryotes harbor two isoform PL kinases, encoded by pdxK and pdxY genes. Notably, the enzyme coded by the pdxY gene lacks catalytic activity, and subsequent references to PL kinase typically pertain to the enzyme coded by the pdxK gene.23,24
Vitamin B6 functions as an enzymatic co-factor, participating in more than 140 biochemical reactions. These reactions encompass aldol cleavages, transamination’s, racemization’s, eliminations, decarboxylation’s, and replacement reactions. In addition to amino acid biosynthesis and degradation, vitamin B6 is also involved in the metabolism of sugars and fatty acids.25Vitamin B6 is composed of a set of six related compounds: pyridoxal (PL), pyridoxine (PN), pyridoxamine (PM), along with their respective 5′-phosphates (PLP, PNP, and PMP) (Figure 2).
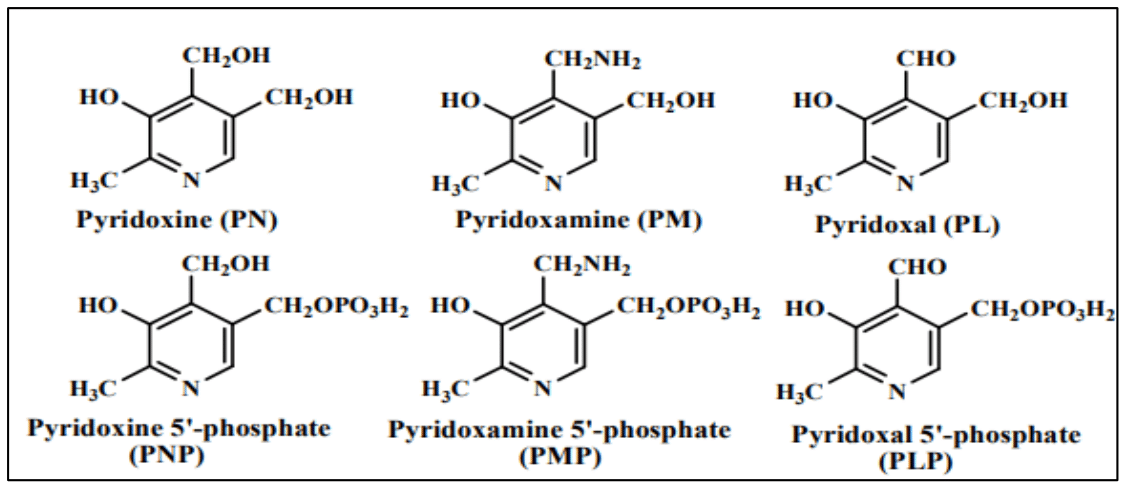
Figure 2 Chemical Structures of the six B6 vitamers- pyridoxine (PN), pyridoxamine (PM), pyridoxal (PL), along with their respective phosphorylated derivatives (PNP, PMP, and PLP).
The variations among them lie in a variable group present at their 4-position, with PN carrying a hydroxymethyl group, and PL and PM possessing an aldehyde and an amino methyl group, respectively. Additionally, all three B6 vitamers undergo phosphorylation by a kinase, a prerequisite for their function as cofactors in enzymatic reactions. An integral element of vitamin B6 metabolism is the newly identified PLP-binding protein (PLP-BP), found widely across all life kingdoms. While lacking catalytic activity, this protein plays a crucial regulatory role in maintaining PLP homeostasis.26
Notably, PLP is a highly reactive aldehyde that readily forms compounds with amino and thiol groups. Thus, it is essential to uphold a proper equilibrium among B6 vitamers within the cell, ensuring that the intracellular-free PLP concentration remains below toxic levels yet sufficient to saturate all PLP-dependent enzymes. Although pyridoxamine-5’-phosphate (PMP) has been recognized as a co-factor, the biologically most active form is pyridoxal 5’-phosphate (PLP). Mammals lack the ability to synthesize PLP from scratch and instead rely on dietary intake of pyridoxine (PN), pyridoxal (PL), and pyridoxamine (PM). The metabolic cycle of vitamin B6 in mammals is illustrated in (Figure 3).
This process includes the action of PL kinase, which phosphorylates PL, PN, and PM, leading to the formation of PLP, PNP, and PMP, respectively (reaction 1, Figure 3). Subsequently, PNP oxidase catalyses the conversion of PNP and PMP into PLP (reaction 2, Figure 3).
In bacteria, plants, and fungi, the vitamin B6 cycle operates alongside a de novo biosynthetic pathway, originating from common cellular metabolites. Additionally, there exists a salvage pathway during protein degradation for synthesizing pyridoxal 5’-phosphate (PLP). In this salvage pathway, PMP, PNP, and PLP undergo dephosphorylation by phosphatases, transforming into PM, PN, and PL. Subsequently, oxidase and/or kinase enzymes convert them back to PLP, as depicted in (Figure 3).
Numerous studies have demonstrated that various phosphatases can efficiently carry out this task, boasting high turnover numbers.26,27 Notably, the kcat values of phosphatases are 30-fold higher than those of PL kinase and even surpass the turnover rates of PNP oxidase by several folds. The cellular content of PLP is intricately dependent on the activities of PL kinase, PNP oxidase, and phosphatases. PLP synthesized by PL kinase or PNP oxidase serves as a crucial cofactor for several apo-B6 enzymes, facilitating the formation of catalytically competent holoenzymes (reaction 3, Figure 3).25,26 Consequently, both PL kinase and PNP oxidase play pivotal roles in ensuring an adequate supply of PLP to meet the cellular requirements.
The Institute of Medicine's Food and Nutrition Board in the United States suggests varying levels of vit B6 intake. 1.3 mg is recommended for young adults, and the suggested intake increases to 1.7 mg for adult males, with a potential increase to 2 mg for lactating women. Potatoes, a dietary staple for many, prove to be an outstanding source of this vitamin. Baked potatoes and potato chips, for instance, can provide up to 23% and 60% of the recommended values per 100 g, respectively as demonstrated in Table 1.
Additional food items such as meat, nuts, bananas, and eggs also provide substantial amounts of vitamin B6 (Table 1). It's noteworthy that vitamin B6 is heat-stable, and its content remains unaffected by cooking or frying. In a generally healthy population with access to a well-balanced diet, vitamin B6 deficiency is uncommon. However, specific demographic groups, particularly individuals aged 45 and above, may exhibit lower levels of pyridoxal phosphate (PLP) in their plasma. In such cases, a diet fortified with vitamin B6 may be beneficial. Individuals with alcohol dependence,28 obesity,29 and pregnant women30 often exhibit diminished PLP concentrations. A deficiency in vitamin B6 is linked to various pathological conditions, such as chronic renal illness, end-stage renal diseases, and other kidney ailments.30
|
Food Source |
VitB6 [mg/100 |
Food Source |
VitB6[mg/100g] |
|
4.07 |
0.83 |
||
|
3.75 |
0.8 |
||
|
1.235 |
0.47 |
||
|
1.157 |
0.367 |
||
|
1.07 |
|
0.257 |
|
|
1.04 |
0.121 |
Table 1 USDA Food Composition Database-Selected Foods with VitB6 Content
(https://www.nal.usda.gov/sites/default/files/page-files/Vitamin%20B-6.pdf)
A vitamin B6 deficiency can also result from malabsorption disorders like celiac disease, inflammatory bowel conditions including Crohn's disease and ulcerative colitis,31 and some hereditary illnesses like homocystinuria.32 Those with rheumatoid arthritis frequently display reduced vitamin B6 concentrations, which tend to decrease with the severity of the disease.33
Moreover, the availability of PLP may be reduced by the use of certain medications, such as contraceptives and natural substances.34,35 Vitamin B6 supplementation has the potential to reverse the symptoms of PLP deficiency associated with the conditions mentioned earlier. Vitamin B6 supplements are recognized for their ability to alleviate premenstrual syndrome symptoms36 and address nausea, vomiting during pregnancy,37 and carpal tunnel syndrome.38
Unfortunately, a significant portion of the general population (28–36%) uses vitamin B6 supplements even when not necessary. Maintaining the proper balance of vitamin B6 is crucial, as several reports suggest that an excess of this vitamin can be neurotoxic. Large doses, particularly exceeding 200 mg per day, have adverse effects, primarily impacting the peripheral nervous system39 (Table 1).
Vitamin B6 and cancer risk
Vitamin B6, with its antioxidant properties, holds promise for cancer prevention and treatment. However, the effects of vitamin B6 supplementation on tumor development are controversial.18 Understanding this controversy requires considering that pyridoxal phosphate (PLP), a cofactor in crucial biosynthetic pathways, is vital for cell proliferation. Hence, vitamin B6 availability influences oncogenesis and tumor progression. Interestingly, until the early 1980s, limiting vitamin B6 was considered a potential cancer therapy.18 However, a substantial adverse relationship between PLP blood levels, dietary vitamin B6 consumption, and cancer has been demonstrated by thorough analysis encompassing several cases.40,41.
Vitamin B6 deficiency is strongly associated with increased risk, notably in gastrointestinal and lung cancers.42,43 This reveals vitamin B6's intricate role in cancer, serving as both an antioxidant for prevention and an essential micronutrient crucial for cell growth. Patients with cancer may have lower vitamin B6 levels, which might lead to weakened immunity and increased need for tumor cell biosynthesis.
Genetic regulation of vitamin B6 metabolism in relation to cancer
The expression of genes involved in pyridoxal phosphate (PLP) recycling is strongly associated with cancer, and vitamin B6 plays a critical role in cell proliferation. Among the seven genes selected from a pool of 6487, the PNPO gene, encoding pyridoxine 5'-phosphate oxidase, stood out for its prognostic value in colorectal cancer patients. PNPO expression was shown to be much higher in colorectal cancer tissues when compared to nearby normal tissues, suggesting that it plays a role in the formation of cancer.44 Further investigations have revealed similar connections between salvage pathway enzymes and various tumor types. For instance, studies by Zhang and co-workers showed that overexpression of PNPO is associated with enhanced cell migration, invasion, and proliferation, and that it contributes to the advancement of ovarian surface epithelial malignancies.45
In vitro experiments showed that silencing PNPO induced cell apoptosis and inhibited tumor formation in vivo.
Interestingly, the regulation of PNPO expression appears to be influenced by factors such as pyridoxal phosphate (PLP) levels and transforming growth factor-β. Epithelial ovarian cell proliferation was seen to be inhibited by PLP supplementation, which also suppressed PNPO protein expression. Additionally, PNPO overexpression in breast invasive ductal carcinoma correlated inversely with overall patient survival.46
Knockdown of PNPO in breast cancer cells resulted in decreased proliferation, migration, invasion, and colony formation, along with cell cycle arrest and apoptosis induction. PDXK exhibits upregulation in non-small-cell lung cancer (NSCLC), as evidenced in a study.47 Remarkably, PDXK's increased protein levels in the tumor did not correspond with its mRNA levels, indicating that PDXK expression is post-translationally controlled. Additionally, PDXK is abundantly expressed in myeloid leukemia cells, where its depletion exerts an anti-proliferative effect not rescued by pyridoxine (PN) or pyridoxamine (PM).8
Leukemia proliferation is supported by plasma vitamin B6, as evidenced by the pharmacological suppression of PDXK by isoniazid or the more targeted 4'-O-methylpyridoxine (gingko toxin), which mimics the effects of genetic depletion.8 On the other hand, PDXK knockdown shields human non-small cell lung cancer cells from the cytotoxic effects of several substances, most notably the chemotherapeutic medication cisplatin. Interestingly, pyridoxine (PN) increases DNA damage, which enhances the anti-tumor activity of cisplatin, provided PDXK is present. These findings are explained by the pharmacokinetic action of vitamin B6, which promotes cisplatin's intracellular build-up.48
In summary, Vitamin B6 has a multifaceted effect on cancer, regulating cell proliferation, immune response modulation, and DNA damage prevention (Figure 4). The diverse roles of vitamin B6 in different cancer contexts underscore the need for a comprehensive examination to understand its multifaceted effects.
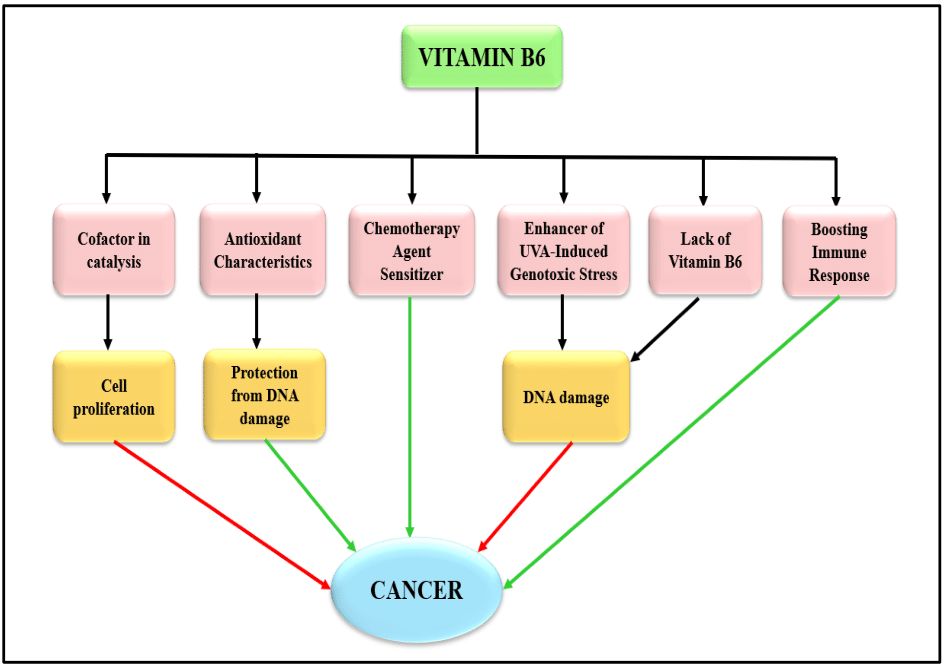
Figure 4 Connections between Cancer and Vitamin B6. Red arrows indicate an impact that promotes cancer, whereas green arrows show a preventative effect against cancer.
Influence of inadequate vitamin B6 levels on DNA metabolism
Vitamin B6 deficiency significantly influences DNA damage and repair processes. Pyridoxal phosphate (PLP), a cofactor of serine hydroxymethyltransferase (SHMT), is crucial for the synthesis of thymidylate the primary source of one-carbon units in metabolism. PLP deficiency can reduce SHMT activity, leading to the misincorporation of uracil in DNA.49 Another enzyme dependent on both PLP and folate, glycine decarboxylase, is essential for purine synthesis and, consequently, DNA metabolism.50
The impact of low vitamin B6 levels extends to the formation of micronuclei in animal models51 and patients with inflammatory bowel disease,52 emphasizing its role in maintaining genomic stability. Vitamin B6 affects DNA through various mechanisms, including the suppression of endothelial cell proliferation and angiogenesis by inhibiting DNA polymerase and DNA topoisomerases.53
In hepatoma cells, pyridoxine induces the expression of insulin-like growth factor-binding protein 1 through the ERK/c-Jun pathway.54 Furthermore, it plays a role in upregulating the expression of p21 via p53 activation in various cancer cells and mouse colon, highlighting its multifaceted influence on DNA regulation.55
Ensuring the effective assimilation of vitamins is vital for preserving genome stability. However, it's equally crucial to acknowledge the influence of an individual's genetic makeup on the ability to absorb, transport, and metabolize vitamins. This may have an effect on the intracellular vitamin B6 level, which may or may not correspond to the amounts and distribution of vitamin B6 in the blood
Understanding vitamin B6 and its associated enzymes is employed in treating diseases at three levels: supplementing with vitamin B6, targeting specific enzymes in the vitamin B6 biosynthesis pathways, and focusing on PLP-dependent enzymes. Given the crucial role of vitamin B6 in neurotransmitter biosynthesis, it is conceivable that various neurological disorders, including Parkinson’s, Alzheimer’s, epilepsy, autism, and schizophrenia, are influenced by the availability of pyridoxal phosphate (PLP).39
For pyridoxine-dependent epilepsy (PDE), characterized by recurrent neonatal or infantile seizures, high doses of PLP have been found to alleviate symptoms.56 While there are currently no specific dosing recommendations, typical long-term PLP doses prescribed remain within the established upper limits (UL).57PDE patients receiving doses exceeding 500 mg/day are carefully monitored for potential adverse effects such as sensory neuropathy.57
PDE is attributed to mutations in the ALDH7A1 (Antiquitin) gene within the lysine degradation pathway, resulting in the accumulation of the lysine intermediate L-aminoadipate-semialdehyde (AASA) and its cyclic form, 1-piperideine-6-carboxylic acid (P6C).58
P6C has the capacity to react with and deactivate PLP. The hypothesis suggests that the subsequent depletion of PLP in the brain leads to overexcitement and epileptic seizures due to the reduced PLP-dependent biosynthesis of the inhibitory neurotransmitter γ-aminobutyric acid (GABA).32 A zebrafish model for PDE has been developed through the generation of an aldh7a1 knockout line, providing researchers with a valuable tool for studying this disease.59 In another instance, dopamine deficiencies are frequently implicated in the primary symptoms of Parkinson’s disease.39
Examining neurons in the substantia nigra region of the human brain revealed the differential expression of four genes, including PDXK.39,60 Some discussions suggest that if PDXK is up-regulated in dopaminergic neurons, the primary source of dopamine in the midbrain, it could potentially alleviate certain symptoms of Parkinson’s disease, although this specific approach has not been investigated.60 However, scepticism exists regarding these findings, as the studies were conducted in isolated populations where a high degree of inbreeding due to local community marriage may occur. Additionally, there was an absence of the same differential expression of the four genes, including the pyridoxal kinase locus, in these populations.60–62
In the subsequent paragraphs, we will delve into the intersection of pathogens and vitamin B6, an emerging field that holds promising opportunities for developing new strategies to prevent infections. In recent developments, the relationship between pathogens and vitamin B6 (vitB6) has emerged as a promising field, offering potential strategies for infection prevention.63
VitB6, known for its role in pathogen-host interactions, was initially identified in the phytopathogen Cercospora nicotianae.64,65 This fungus, using a photosensitizer, produces reactive oxygen species (ROS) crucial for penetrating host plant tissue. This marked the initial identification of vitamin B6 as a potent antioxidant capable of effectively protecting cells against rising reactive oxygen species (ROS).66 Furthermore, it represented the first recognition of its pivotal role in the intricate interplay between hosts and pathogens.
Plants also leverage vitB6 to reduce infection risks. Arabidopsis mutants with impaired vitB6 pathways showed increased susceptibility to bacterial and fungal infections, suggesting a role in cellular defense responses.67 In another example, the bacterium P. luminescens, symbiotic with the nematode H. bacteriophora,68 exhibited reduced pathogenicity in mutants affecting vitB6 biosynthesis, emphasizing the importance of vitB6 for a defined metabolic rate in supporting either mutualistic relationships or pathogenic abilities. A genetic screening for P. luminescens mutants with reduced pathogenicity identified a mutation in PdxB, an erythronate-4-phosphate dehydrogenase, a component of the DXP-dependent de novo pathway.69
PdxB mutants exhibit impaired overall growth, a deficiency that can be alleviated by adding pyridoxal-5'-phosphate (PLP) to the growth media. Additionally, growth restoration is achievable not only with PLP but also with non-phosphorylated vitB6, indicating the bacterium's effective vitB6 uptake systems and a functional salvage pathway.69
While the specific impact of vitB6 on pathogenicity remains unclear, maintaining a defined metabolic rate with sufficient PLP levels is considered essential to support either the mutualistic lifestyle or the capacity to infect other organisms.69 The correlation between vitB6 biosynthesis and pathogenicity extends to other bacteria, including Helicobacter pylori, Streptococcus pneumoniae, and Mycobacterium tuberculosis.70,71 In H. pylori, vitB6 deficiency impairs virulence and flagellar glycosylation, leading to immobile bacteria.70
Similarly, S. pneumoniae with compromised vitB6 biosynthesis genes demonstrated attenuated infection rates in a mouse model.72These findings underscore the significance of vitB6 in bacterial pathogenicity and suggest potential avenues for developing novel strategies to combat infections. The loss of the de novo pathway in the tuberculosis-causing bacterium, M. tuberculosis, hinders its persistence in host tissue.71
Interestingly, besides directly affecting vitB6 biosynthesis, supplementing vitamin B6 has shown promise in enhancing tuberculosis treatments. For instance, vitamin B6 inhibits the binding of MtbLrpA to DNA, a transcriptional regulator crucial for M. tuberculosis persistence.73This suggests that targeting the vitB6 pathway could be a potential drug strategy against these bacteria and other pathogens. Since humans lack the de novo vitB6 biosynthesis pathway, this approach is promising, offering specificity to pathogens without harming the host and interfere with the pathogen's metabolic processes to a degree that prevents its survival, thereby reducing or eliminating the pathogen load for the host's recovery. Targeting PDX proteins and specific PLP-dependent enzymes crucial for pathogen viability could satisfy both conditions.
In this regard, malaria, being a eukaryotic pathogen, stands out as a prospective candidate for innovating new anti-malarial drugs through the exploitation of the de novo vitamin B6 pathway. Malaria is a potentially fatal illness that mostly affects equatorial regions and continues to be a major worldwide health problem. The World Health Organization (WHO) recorded 214 million new cases and 438,000 deaths from malaria in 2015.74
Due to Plasmodium falciparum's growing resistance to current anti-malarial medications, the fight against malaria is becoming more difficult. Researchers consistently explore novel drug targets, as highlighted in a 2014 review that identified PLP-dependent enzymes such as ornithine decarboxylase, P. falciparum aspartate aminotransferase, and SHMT as promising therapeutic targets.75,76
However, the challenge lies in avoiding adverse effects on corresponding host enzymes. The suggestion of targeting PLP-related molecules in the Anopheles vector, like 3-hydroxykynurenine transaminase, has been made; however, the lack of adequate structural information poses a challenge to drug design. While there might be other promising targets, dismissing vitB6-related targets for novel treatments against P. falciparum and other pathogens could be an oversight.
The PDXK gene encodes pyridoxal kinase (PDXK), a vitamin B6-dependent transferase enzyme that is essential for leukemic cell proliferation. When its activity is disrupted, it leads to perturbed metabolism, resulting in diminished levels of nucleotides and polyamines. This comprehensive review has unveiled the pivotal role of Pyridoxal kinase (PDXK) and vitamin B6 metabolism across diverse domains, ranging from oncology to infection prevention. The exploration of PDXK as a central player in vitamin B6 metabolism has illuminated its potential as both a biomarker and therapeutic target in the complex landscape of cancer. The association between dysregulated PDXK expression and tumorigenesis emphasizes its promise in innovative cancer therapies. The intricate interplay between vitamin B6, DNA damage, and cancer risk underscores the critical importance of maintaining balanced vitamin B6 homeostasis. Epidemiological insights have revealed nuanced relationships between inadequate vitamin B6 levels and heightened susceptibility to various cancers, underscoring its indispensable role in cellular processes, immune modulation, and genomic stability.
Beyond its implications in cancer, the review has elucidated the broader consequences of imbalanced vitamin B6 homeostasis, linking it to various pathological conditions and emphasizing its significance for overall health. The latter sections of the review have explored the potential of vitamin B6 in novel drug development, particularly in the context of pathogen interactions. Targeting PDX proteins and specific PLP-dependent enzymes in pathogens, especially in malaria, holds promise for ground-breaking advancements in anti-malarial drug development.
In the field of oncology, the exploration of PDXK modulation as a novel strategy presents a compelling avenue. The potential synergies with existing cancer therapies offer an exciting prospect for improving treatment outcomes while minimizing adverse effects.
In conclusion, the multifaceted roles of PDXK and vitamin B6 in cellular processes, DNA metabolism, and pathogen interactions not only expand our understanding of fundamental biological mechanisms but also pave the way for the development of targeted therapeutic interventions in the fields of oncology and infectious diseases. As the scientific community continues to unravel the intricacies of PDXK and vitamin B6, the potential for ground-breaking advancements in medicine becomes increasingly evident.
Disclosure statement
During the preparation of this work, the authors used Chat GPT, in order to improve scientific writing and correct grammatical mistakes. After using this tool, the authors reviewed and edited the content as needed and takes full responsibility for the content of the publication.
The authors declare no relevant financial or non-financial interests to disclose.

©2024 Banerjee, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.