eISSN: 2473-0815


Review Article Volume 4 Issue 3
Consultant, Endocrinology, Metabolism, Chief Medical Officer, EQ Biosciences, USA
Correspondence: David A Westbrock, Consultant, Endocrinology, Metabolism, Chief Medical Officer, EQ Biosciences, USA, Tel 937-470-5405
Received: July 17, 2016 | Published: March 23, 2017
Citation: Westbrock DA. Estrogen metabolites in the etiology of hormone related cancers: possible role of anti-oxidants in prevention. Endocrinol Metab Int J. 2017;4(3):63-68. DOI: 10.15406/emij.2017.04.00086
The goal of this review is to demonstrate the role of metabolic variations in estrogen metabolism and polymorphisms in enzymatic control in the oncogenesis of breast and prostate cancer and Parkinsonism. Further biochemical associations with these cancers are demonstrated. The evidence for interference of oncogenic metabolites by certain anti-oxidants is reviewed. The role of endogenous hormones particularly estrogens in the etiology of several cancers has been postulated for several decades. Benzene has long been associated with leukemia. The benzene ring which includes estrogens, benzene and dopamine are metabolized to ortho-quinones which are highly electrophilic and have been shown to be depurinating to DNA. These metabolites are known as estrogen adducts (EA). Estrogen is metabolized in steps involving formation of hydroxyl-estrogens further oxidized by CYP 450 enzymes or peroxidases or by catechol o methyl transferase, and finally by glutathione. In vivo studies demonstrate induction of altered DNA due to purine base knockouts leading to malignant transformation in MC-10 mouse cell lines. Further evidence reveals these changes are not estrogen receptor dependent. Statistically higher urinary levels of EAs have been demonstrated in women with a history of breast cancer and that of men with prostate cancer and lymphoma, as well as in malignant human breast epithelial tissue. In a genetic population of high risk cancers, manifested by BRCA 1 and 2 alterations, DNA double stranded breaks are increased when mice cells are incubated with EAs. BRCA mutations are responsible for induction of substrate metabolites 2-OH E2 and 4 OH E2 by failure of control of cytochrome P450 enzymes (aromatizes) as well as inhibition of quinone oxoreductase which reduces estrogen quinones to lessen formation of EAs. Adducts have an important role in neurotransmitter metabolism, for example in the formation of dopamine quinone DNA adducts. Loss of dopamine in the substantia nigra and metabolic changes in dopamine metabolism may have a role in the pathophysiology of Parkinson’s disease. At lower pH ranges dopamine (DA) quinone is metabolized to lead to increasing competitive increase in electrophilic attraction to nucleotide adducts DA-6-N3 Ade and DA 6-N7 Gua which lead to mutagenesis and degeneration in cells of the subsantia nigra. Further research has demonstrated that antioxidants, resveratrol and N-acetyl cysteine (NAc) inhibit formation of EAs in cultured mammary tissue of MC-10 mice, by enhancement of quinone reductase and inhibition of attachment of quinones to DNA purines through glutathione induction. In the case of DA metabolism, reduction of quinone nucleotide activity was demonstrated with the use of lipoic acid, NAc, melatonin and resveratrol. Further clinical trials and epidemiologic studies in humans with described cancers and Parkinson disease are necessary to define these models and prevention in the future.
Keywords: estrogen, oncogenic, metabolites, hormone, cancer, etiology, NAc, EAs, parkinson’s disease
Much controversy exists concerning the risk of breast cancer and other cancers as well as general health benefits in women utilizing hormone replacement following menopause. In the past, several studies have shown benefits of estrogen (and progesterone) use,1,2 or lack of cancer risk,3 while others have shown a relative increased risk.4-6 Multiple etiologies, in addition, have been posited in regard to other malignances. Basic research aside little has been documented in clinical literature regarding the biochemistry behind how estrogen and/or its metabolic byproducts may have a role in genetic mutations and cancer induction. Reports relating benzene to mutagenesis date to the 1920s with animal studies first reported in 1979.7 Further speculation exists based on the effects of endocrine disruptors, such as pollutants not as directly carcinogenic but as agents that disrupt estrogen balance and increase formation of EAs.
Further, Cavalieri8 investigating the role of derivatives of the steroid ring with its aromatic hydrocarbon bonds, the basic structure of steroid chemistry, studied the association of endogenous estrogen as a carcinogenic agent and formulated new science wherein metabolic products of estrogen metabolism were identified. The conversion to highly electrophilic estrogen quinones leads to attachment to both nucleic acids bases adenine and guanine to cause knock out genes and resulting genetic mutation. Further studies,9-12 over the past 40 years have led to the association of certain cancers, particularly breast, prostate, lymphoma, and ovarian with EAs. Some non-malignant disorders such as Parkinson’s disease and Alzheimer’s dementia are also being studied.
With the identification of the induction of mutagenic cells, further studies have measured these metabolites in patients with cancer and individuals at high risk. The next step has been to develop agents capable of blocking the synthesis or enhancing metabolic disposal of estrogen quinones as a final metabolic pathway. A unique agent, a combination of antioxidants, has been developed to ablate estrogen quinone formation and the induction of depurinating EAs.
Chemistry of estrogen carcinogenesis
Aromatase, a cytochrome p450 (CYP 19) enzyme converts androstenedione and testosterone to estrone (E1) and estradiol (E2) respectively. Aromatase inhibitors are commonly used as preventative measures following radiation, surgery and chemotherapy for breast cancer. E1 and E2 are then hydoxylated at either position 2 or 4 (2-OHE2 and 4-OHE2) and methylated via catechol-O-methyl transferase (COMT) see Figure 1. Additional metabolism is catalyzed by peroxidases and subsequent conversion to quinones, or a reversible reaction via CYP reductases. Further metabolism may prevent final quinone production by glutathione conjugation. Steps to prevent final quinone and estrogen adduct formation then lie at the COMT, glutathione and final formation of adduct into DNA. Reduction of quinones and adducts have been demonstrated by several agents (Figure 2).
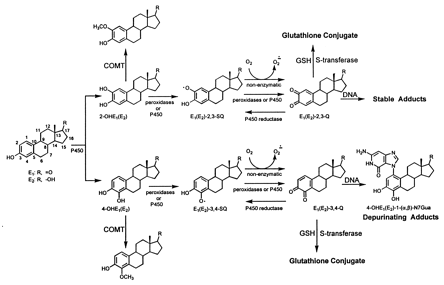
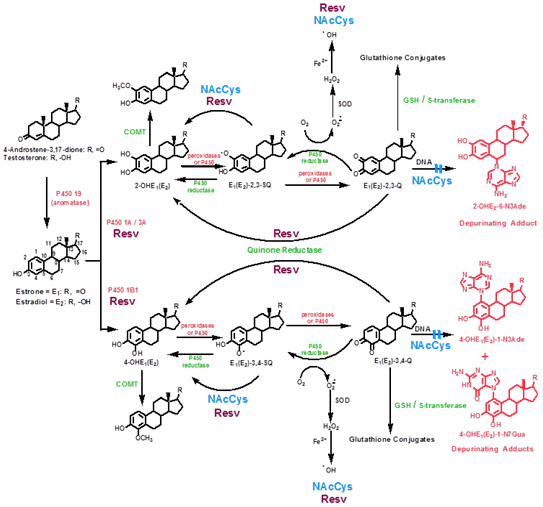
Estrogen induction of breast and ovarian cancer
A connection between estrogen and breast cancer has been presumed for many decades, dating from the first noted remission of breast cancer following oophorectomy.13 Risk factors, among others, include obesity, increased endogenous estrogen, androstenedione and testosterone levels. Metanalysis14 has demonstrated an increase in relative risk of breast cancer related to use of endogenous estrogen. The Women’s Health Initiative (WHI)15 showed an increased relative risk of hormone replacement therapy compared to controls, but as a caveat, not in those women on estrogen alone. Studies prior to WHI have been conflicting.
In addition, two identifiable genetic loci (BRCA1, BRCA2) have been identified on chromosome 17 and 13 respectively. Although these genes are responsible for less than 10% of breast cancers, the relative risk of breast and ovarian cancer increases to between 20%-80%, increasing with age. Both are tumor suppressor genes, and mutations induced by estrogen adducts may have a role in cancer risk for both breast and ovarian cancer.16 A study of urine and saliva in 33 women with ovarian cancer compared to age matched controls revealed a 2½ fold higher ratio of estrogen adducts to total estrogen metabolites.17
In vitro studies of a human mammary cell line which is estrogen receptor negative (MCF10F) showed tumorigenesis when incubated with estradiol (E2) following injection of cultured cells into a strain of immune-deficient mice.18 In this cell line, then, mutagenesis is not estrogen receptor dependent. Genetic alterations were identified in multiple chromosomes, some similar in human breast, liver, bladder, renal cell, prostate and lymphoma.
Genetic alterations influencing estrogen metabolism carcinogenesis
A study matching controls with 503 epithelial ovarian cancer patients revealed that the joint effects of alterations of four separate genes involved in the formation of catechol estrogen (COMT, SULT1A1, CYP1A1 and CYP 1B1) were significantly associated with ovarian cancer risk.19 Similar findings in 150 age matched controls compared to 50 breast cancer patients showed a fourfold cancer risk in those with higher activity genotypes for CYP 1A1, CYP 17, and COMT.20
Furthermore, polymorphisms of quinone reductase (NQ1) and catechol-o-methyl transferase (COMT)21,22 have been demonstrated in breast tissue of women with breast cancer leading to lower alternative metabolism of estrogen quinones. Additionally, lower levels of glutathione which further metabolize estrogen quinones are found to be in lower levels compared to non cancer affected individuals.23
Role of depurinating adducts
Further, additional studies24 revealed the ability of 4-OHE2 to transform MCF10F cells which was not affected by inclusion of the synthetic estrogen modulator (SERM) tamoxifen into cell media. Depurinating adducts of guanine and adenines were then found following treatment. Human studies,25 in women treated with aromatase inhibitor blocking conversion of testosterone to estrogen showed a lower incidence of breast cancer in the contralateral breast compared to anti- estrogen tamoxifen indicating the effect of estrogen or its metabolite(s) and not estrogen receptors. Analysis of transgenic ER-alpha mice (ERKO/wnt-1) showed mammary tissue containing glutathione conjugates of E1 (E2)-3,4-Q but no conjugates formed by COMT methoxalation,26,27 signifying decrease in E1 (E2) -3,4-Q metabolism of quinones by COMT metabolism and increase in quinone production and adduct formation. Women with previously diagnosed breast cancer were found to contain fourfold higher levels of 4-OH E2 in normal breast tissue than women without cancer.28 Analysis of urinary excretion of estrogen adducts reveals higher levels in women with a history of breast cancer or at high risk.29
Estrogen adducts induced by CYP mutations in Prostate cancer
Early studies correlating the risk of prostate cancer to sex steroid levels included a prospective analysis of the Physicians Health Study30 in which 222 males with prostate cancer were compared to 390 controls matched for age, smoking status and length of follow-up. Correlation of risk was associated with higher levels of testosterone, but not estrogen levels, but estrogen levels in sera31 are not an indication of estrogenic effect since estrogen may be synthesized intra-prostatically and correlates poorly with serum level. A later study,32 revealed that CYP 19A1 may be elevated as much as 30 fold in metastatic cancer tissue and further evidence by a 1983 study showed that genetic polymorphisms (CYP 1B1, CYP 19) were found to correlate with cancer risk.33 Two studies revealed the association of urinary levels of depurinating estrogen adducts in men with prostate cancer. Mean levels of adducts were greater than double in 14 men with prostate cancer34 (X= 57.3) vs. 125 case controls (x= 23.4).35
BRCA genes associated with alterations in estrogen metabolism
The BRCA genes are tumor suppressor genes36 and when dysfunctional as in the case of mutations of BRCA 1 and 2 confer a risk of up to 85% for breast cancer and 20% of ovarian cancer Savage et al.,37 Demonstrated that BRCA1 not only is responsible for repair of damaged DNA but has an essential part in regulating estrogen metabolism.38 Estrogen metabolites 2-OH E2 and 4-OH E2 (initial metabolites of estrogen metabolism following aromatization and induced by CYP 450 1A,2A,3A and 1B1) induced DNA double strand breaks (DSBs) in both estrogen receptor negative MCF-10A mice breast tissue cells as well breast tissue from MCF-7 estrogen receptor positive cells. These DSBs were demonstrated to occur in 25-30% greater numbers of cells following incubation of cells with 2-OH E2 and 4-OHE2. Further indications were that intact BRCA1 repairs damaged DNA induced by estrogen quinones. Additionally, normally BRCA1 inhibits CYP 1A1 [39] thus decreasing formation of hydroxy estrogens and adduct formation. Additionally, Savage showed an increase in CYP3A1 and reductase (NQO1) which reduces quinones to mono or di-hydroxy estrogens.
Investigators,40,41 demonstrated secondary mutations in BRCA1 and 2 in platinum chemotherapy treated patients, in which telomeres were repaired in the altered genes (BRCA1,2) leading to decrease in apoptosis, further chromosomal double strand breaks (DSBs) and drug resistance.
Role of catechol dysmetabolism in the etiology of Parkinson’s Disease
Dopamine, one of three major neurotransmitters and its progressive loss in the subsantia nigra via oxidative stress is the major feature of Parkinson’s disease (PD).42 It is formed in several steps from phenylalanine and subsequently to dihydroxy-phenylalanine (L-DOPA) and further decarboxylated to dopamine. Degradation is complex and may form dopamine quinone by subsequent oxidation at the 3,4 position via tyrosinase to form dopamine -0- quinone. These quinones generate superoxide radicals which alter the functionality of multiple components. In addition, these quinones may also form neurotoxic molecules such as salsolinol furthering oxidative stress and mitochondrial dysfunction. Negative effects on cell metabolism are further generated by a small protein (alpha- synuclein) found in Lewy bodies, a feature of PD and responsible for inhibition of dopamine synthesis.
Further research43 illustrates that at low pH dopamine quinones form depurinating adducts such as dopamine -6N-N3 adenine and dopamine-6-N7 guanine, and may do further damage to neurons from genetic alterations. Investigation of biomarker excretion was performed in 20 PD patients on dopamine agonists compared to 40 control pts without PD.44 Of estrogen metabolites analyzed, significantly higher levels (p=.004) of thiol (glutathione mediated) and methoxy (COMT derived) conjugates were noted in controls.
Additionally, ratios of estrogen adducts to the total of other estrogen conjugates were also higher (p=.008). Several genes mutations such as the Parkin gene have been implicated in PD patients.45 Together with the bio- toxicity of quinone conjugates of dopamine, a strong case for these adducts in the etiology of Parkinson’s disease can be made.
Agents that inhibit estrogen adduct formation
Antioxidants have been recognized that prevent the formation of quinone generation and the synthesis of adducts. Those either facilitating alternative pathways of estrogen metabolism, either via the glutathione pathway or through methylation by COMT, have been shown to ablate serum and urinary levels of adducts.46
Resveratrol (Res), categorized as a stilbene, is a tri hydoxylated molecule with a bi-phenolic ring. It is found in a wide variety of plants, grapes, legumes, eucalyptus and peanuts among many others. It has been widely promoted for anti atherogenic effects, but has been found to be anti inflammatory and anti-neoplastic.47 It exerts its effects in a number of ways including apoptosis by its effects on multiple signaling proteins. It has shown anti-proliferative effects on multiple cells including lymphoma, myeloid, breast, colon, pancreas, stomach, prostate cancer and others. In studies involving ER cell lines MCF-7, MCF-10F and MDA-MB-231, among several signaling points, Res was shown to inhibit CYP1A1 expression,48 as well as CYP1B1 and inducing enzyme NQO149 (Figure2). Additional effects are seen at the conversion of E1 (E2) to 4-OHE1 (E2) and by reacting with semi-quinones to reduce them to catechol estrogens which are then methylated via COMT, on the acceptance of the oxygen radical, and on DNA polymerase.50
N-acetylcysteine (NAc) has been shown to ablate estrogen adducts via similar pathways and were shown51 and by additionally revealing increased levels of glutathione conjugates and increasing affinity to estrogen quinones and decreasing affinity to nucleic acids to form adducts.
The association of electrophilic products of estrogen metabolism (estrogen quinones) forming a complex with purine bases in DNA have been clearly demonstrated in animal models to be mutagenic and shown to be statistically elevated in patients with breast and prostate cancer and lymphomas. Additional evidence reveals increased estrogen adducts in breast tissue of human breast cancer cells. Although most studies have revealed complex biochemical interactions in vitro and in animal models, further human trials are necessary to study the efficacy of specified anti-oxidant agents to not only reduce the risk of cancers via prevention of DNA damage by eliminating EA formation, but to study the ability of these agents to influence the course of the disease, including individuals with BRCA1 and 2.
Studies are planned to test the clinical course and markers of prostate cancer patients who choose to forego therapy. In addition, further epidemiologic studies of BRCA 1 and 2 gene positive individuals and correlation with EAs are needed, as well as the potential role of specific anti-oxidants in the prevention of breast, ovarian, prostate cancer and Parkinson’s disease needs to be elucidated. Endocrine disruptors are defined as agents that interfere with the body’s endocrine system and produce adverse developmental, reproductive, neurological and immune effects in this case tumorigenesis and cancer formation.53 Parabens, a preservative in many cosmetic products, has been found to be in very high levels in 20 human breast cancer specimens. Chemicals such as bisphenol A (BPA), phthalates, perchlorate, atrazine and others have been so classified and linked to breast54 and prostate55 cancer. Whether these or other chemicals are linked to estrogen adduct formation is unknown. A genetic link to cancer risk has been shown in patients with BRCA gene alterations, which have been shown to be associated with alterations in estrogen metabolism (EA formation). Any association with environmental chemical exposure relative to association with estrogen adducts awaits future clinical trials. As well, EA formation in BRCA altered individuals should be evaluated since anti-oxidant agents that reduce EAs have been demonstrated and may have an essential role in cancer prevention.
None.
The author declares there is no conflict of interest.

©2017 Westbrock. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.