eISSN: 2473-0815


Research Article Volume 4 Issue 1
1Division of Endocrinology, Metabolic Medicine, Mubarak Al Kabeer Hospital, Kuwait
2Department of Medicine, Faculty of Medicine, Kuwait University, Kuwait
Correspondence: Kamal Al Shoumer, Professor, Faculty of Medicine, Kuwait University, Consultant, Head, Division of Endocrinology, Metabolic Medicine, Department of Medicine, Mubarak Al Kabeer Hospital, Faculty of Medicine, Kuwait University, P O, Box 24923, 13110 Safat, Kuwait, Tel +965 99707539
Received: October 29, 2016 | Published: February 22, 2017
Citation: Al-Shoumer KAS, Nair VS. Changes in acid labile subunit (ALS) and insulin-like growth factor 1 (IGF-1) concentration in hyperthyroidism before and after medical treatment: a prospective controlled study. Endocrinol Metab Int J. 2017;4(2):24-30. DOI: 10.15406/emij.2017.04.00077
Background: The relationship of altered thyroid status with GH and components of insulin-like Growth factor (IGF-1) axis is complex and not fully understood. We aimed to evaluate changes in Acid labile subunit (ALS) and IGF-1 in hyperthyroidism, before and after normalization of thyroid status.
Methods: Thirty-four hyperthyroid patients matched with 36 normal controls were studied. The patients and controls were assessed at baseline and the patients were followed up prospectively and re-assessed after 6 months of treatment with antithyroid drug (ATD) (carbimazole). On each occasion, blood was collected for measurement of ALS, IGF-1, glucose, insulin, intact proinsulin and thyroid function.
Results: Pre-treatment levels of ALS (p=0.012) and IGF-1 (p=0.007) were significantly lower in patients than controls. Five to 10 weeks post-treatment with ATD, all patients were restored to euthyroidism. ALS and IGF-1 levels increased significantly to similar levels of controls after 6 months of ATD. Within the patients, ALS (p=0.049) and IGF-1 (p=0.001) correlated inversely with free T3, whereas, only IGF-1 (p=0.005) correlated inversely with free T4.
Conclusion: These results demonstrate that untreated hyperthyroidism is associated with reduction in ALS and IGF-1. Due to the fact that ALS and IGF-1 were related inversely with thyroid status and their concentrations were normalized with euthyroidism, it would be possible to consider their reductions as helpful markers of altered thyroid status, namely hyperthyroidism.
Keywords: ALS, IGF-1, hyperthyroidism, medical treatment
IGF-1, insulin-like growth factor 1; ALS, acid labile subunit; ATD, antithyroid drug; GH, growth hormone; GHRH, growth hormone releasing hormone; IGFBP, IGF-binding protein; HOMA, homeostasis model assessment; IR, insulin resistance; RAIU, radioactive iodine uptake; CV, coefficients of variation
Thyroid hormones are essential for normal growth and development of many tissues. Hypothyroidism is associated with growth impairment, and hyperthyroidism with the development of a hyper catabolic state and skeletal muscle wasting. Thyroid hormones influence GH synthesis and secretion, and impaired GH responses to many pharmacological stimuli, including GH releasing hormone (GHRH), has been described in patients with thyrotoxicosis.1-10 The most important peripheral mediator of human GH activity is the insulin-like growth factor-1 (IGF-1). In circulation, almost all the IGF-1 are present as 150 kDa ternary complexes comprising of one molecule each of IGF-1, IGF-binding protein- (IGFBP)-3 (the predominant IGFBP in serum) or IGFBP-5, and 85 kDa glycoprotein, the acid-labile subunit (ALS).11-13 The acid-labile subunit is a glycoprotein found almost exclusively in the circulation and produced in the liver under growth hormone stimulation.14-15 Formation of the ternary complexes restricts the IGFs to the circulation, prolongs their half-lives and allows them to be stored at high concentration in plasma to facilitate their endocrine actions and to minimize their local effects due to their intrinsic insulin-like activities such as hypoglycemia.16 The relationship of altered thyroid status with growth hormone changes is well known. However, changes in components of insulin-like growth factor axis including ALS in patients with hyperthyroidism are not well investigated. In view of the lack of comprehensive data and definitive conclusions relating the effects of hyperthyroidism on the serum concentration of IGF-1 and ALS, we decided to perform this study in which we examined the alterations in levels of insulin-like growth factor 1 and Acid-Labile Subunit in untreated hyperthyroid patients before and after medical therapy with antithyroid medications. We further explored the metabolic relations of IGF-1 and ALS in hyperthyroidism.
Thirty-four patients with primary hyperthyroidism and thirty-six normal subjects were studied. The patients and controls were matched for ethnic group, age, sex and body mass index (Table 1). Patients were recruited from the endocrine clinic at Mubarak Al-Kabeer Hospital, Kuwait, and normal control subjects were included in the study if they were free from illnesses and were not taking any medication. The study protocol was approved by the Local Ethics Committee and subjects gave informed written consent. Primary hyperthyroidism was due to Graves’ disease (diagnosed on the basis of elevated free thyroid hormones, suppressed TSH and elevated 24 hour RAIU) and all patients were clinically and biochemically hyperthyroid at baseline (Table 1). It is of importance to mention that the diagnosis of hyperthyroidism doesn’t always create a problem based on clinical findings and thyroid function tests, and in practice no more advanced and expensive tests to aid the diagnosis are needed. However, we have performed RAIU in order to eliminate subjects with all other transient causes of hyperthyroidism due to thyroiditis. No patient or control had other medical illness and none of them was taking any medications other than those mentioned for the patients in the experimental protocol. Women subjects (patients and controls) were pre-menopausal who had regular menses.
Patients |
Controls |
P Value |
|
Total |
34 |
36 |
|
Male: female |
12:22 |
13:23 |
|
Age (years) |
34 (2) |
35 (2) |
NS |
BMI (kg/m2) |
22.5 (0.8) |
23.9 (0.7) |
NS |
Free T3 (pmol/l) |
29.6 (1.8) |
4.6 (0.2) |
0.0001 |
Free T4 (pmol/l) |
83.9 (4.8) |
15.8 (0.6) |
0.0001 |
TSH (mU/l) |
< 0.01 |
1.44 (0.3) |
0.0001 |
Table 1 Clinical characteristics of untreated hyperthyroid patients and con
Serum TSH levels in all hyperthyroid patients were all suppressed below 0.01 mU/l. Normal ranges in our laboratory were 3.3-7.2 pmol/l for free T3, 11.0-24.0 pmol/l for free T4 and 0.27-4.6 mU/l for TSH.
Abbrevations: SEM, values are mean; BMI, body mass index; NS, not significant statistically.
Experimental protocol
Subjects attended the Metabolic Day assessment ward at Mubarak Al-Kabeer Hospital, Kuwait after an overnight fast of 10-12 hours. All women (patients and controls) were studied during the first 10 days of their cycles. Weight, height, pulse and blood pressure were measured. Fasting blood samples were collected at 8 am for the measurement total acid-labile subunit (ALS) and Insulin-like growth factor 1 (IGF-1) and for the measurement of parameters of glucose homeostasis and insulin secretion including glucose, insulin and intact proinsulin. Full blood count, liver, renal and thyroid function tests (free T3, free T4 and TSH) was also assessed. Patients were followed up for 6 months with antithyroid medications [carbimazole (Nicholas, Basel, Switzerland)]. Their fasting blood was then taken at 6 months for the measurement of ALS, IGF-1, glucose, insulin, intact proinsulin and thyroid function.
Laboratory methods
Blood samples were transferred on ice immediately, centrifuged at 2500 rpm at 4°C for 15 minutes and the supernatants were stored at-70°C until analysis. Free T3 and free T4 were assayed using a direct labelled antibody competitive radioimmunoassaytechnique (Amerlex-MABkits, Ortho-Clinical Diagnostics, Amersham, UK) and highly sensitive (hs) TSH was measured utilizing one-step immuno radiometric technique (Ortho-Clinical Diagnostics, Amersham, UK). ALS was determined using a two-step sandwich type immunoassay (ELISA, DSL, Texas, USA) where samples were incubated in microfiltration wells which have been coated with anti-ALS detection antibody labelled with the enzyme horseradish peroxidise. Inter-assay and intra-assay Coefficients of Variation (CV) for ALS respectively were 7.5% and 2.8% and the test sensitivity was 0.7μg/ml. IGF-1 similarly was measured by ELISA (DSL, Texas, and USA). The intra-assay and inter-assay CV were 3.1% and 6.0% for IGF-1 (at 16 ng/ml).
Plasma glucose was measured by an enzymatic colorimetric test using an automatic colorimeter (Hitachi 717, Boehringer Mannheim Gmbh Diagnostica, Mannheim, Germany). Insulin concentrations were measured by radioimmunoassay. Insulin resistance (IR) was calculated from the homeostasis model assessment equation: HOMA-IR=Fasting insulin (mU/l), X fasting glucose (mmol/l)/22.5. This equation constitutes the homeostasis model assessment (HOMA) estimate of IR that has been validated by comparison with results of glucose clamp studies [17]. Intact proinsulin was measured by an ELISA method (DAKO Diagnostica, Cambridge shire, UK). The inter-assay and intra-assay CV respectively were 7.0% and 7.8% for insulin (at 30.7 mU/l), 4.5% and 5.2% for intact proinsulin (at 4.2 pmol/l), 5.0% and 4.1% for free T3 (at 5.9 pmol/l), 3.0% and 3.7% for free T4 (at 17.8 pmol/l), and 10% and 1.6% for hsTSH (at 5.9 mU/l). Hemoglobin, packed cell volume, white cell count, platelets, and liver and renal functions were measured by routine laboratory techniques.
Statistical analysis
Data are expressed as mean ± standard error of the mean or median (range). Data for patients and controls were compared using Mann-Whitney U test or Student’s unpaired t test as appropriate. Correlations between variables were sought using Spearman’s rank correlation coeffient (rho). No normally distributed variables were normalized by log-transformation prior to analysis. P level of less than 0.05 was considered statistically significant.
Subjects and study progress
The baseline data for hemoglobin, white cell count, packed cell volume, platelets, creatinine, total cholesterol, triglycerides, systolic and diastolic blood pressure were within the normal range in all patients and controls. After the initial work up, patients were treated with antithyroid drugs and beta blocker agents where required (propranolol was used for 21 patients in the first 4-6 weeks). The median (range) initial antithyroid (carbimazole) and beta blocker (propranolol) doses were 40 (10-60) mg/day and 80(40-160)mg/day respectively. The maintenance carbimazole dose was 15(10-20) mg/day. After carbimazole administration by 5-10 weeks, all patients had free T3 (5.0±0.6 pmol/l), free T4 (16.4±1.9 pmol/l) and TSH (2.5±0.6 mU/l) levels within the normal range and were similar to controls. These levels were maintained until the end of study at 6 months. No carbimazole side effects were reported.
Baseline ALS levels and their changes after treatment
Untreated hyperthyroid patients had significantly lower ALS concentrations than normal control subjects (patients vs. controls, 15.9±1.5 vs. 20.0±0.7 μg/ml, p=0.012). Levels of ALS increased significantly, after 6 months therapy with antithyroid drugs, to levels (20.1±1.8 μg/ml, p=0.006, compared with pre-treatment level) similar to those of control subjects (Figure 1).
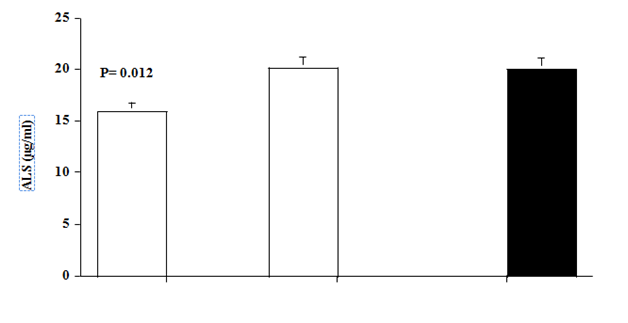
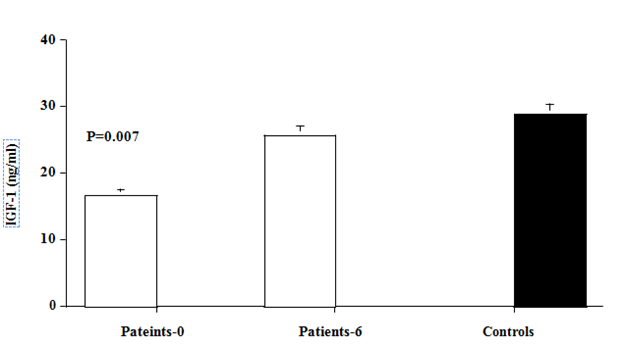
Figure 1 Acid labile subunit (ALS) and Insulin-Like Growth factor- 1 (IGF-1) levels in hyperthyroid patients (Open bars) at baseline and after 6 months of antithyroid therapy, and in matched normal (black bar). Values are mean ±SEM. P values are those between baseline data of patients and controls.
Baseline IGF-1 levels and their changes after treatment
Pre-treatment levels of IGF-1 were significantly lower in hyperthyroid patients compared with normal control subjects (16.7±2.4 vs. 28.9±2.3ng/ml, p=0.007). Levels of IGf-1 increased significantly, after 6 months therapy with antithyroid drugs to levels (25.8±4.2ng/ml, p=0.046, compared with pre-treatment level) similar to those of control subjects (Figure 1).
Baseline glucose, insulin, intact proinsulin and HOMA insulin resistance
Pretreatment fasting glucose (p=0.01), insulin (p=0.007), intact proinsulin (p=0.02) were significantly higher in patients than controls. Patients tended to be more insulin insensitive compared with controls as shown by the calculated HOMA insulin resistance (p=0.07). After 6 months of antithyroid therapy with attainment of euthyroidism, concentrations of fasting glucose, insulin, intact proinsulin and HOMA insulin resistance decreased significantly in the patients and became similar to those of controls (Figure 2).
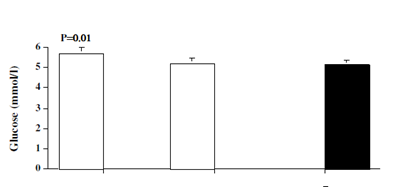
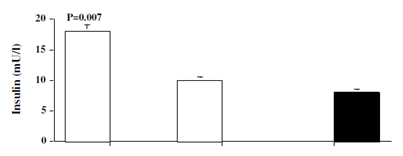
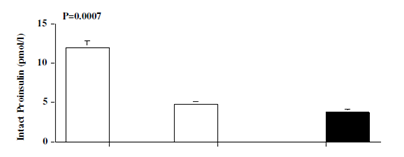
Figure 2 Levels of glucose, insulin, proinsulin and HOMA IR in hyperthyroid patients (open bar) at baseline (patients-0) and after 6 months (patients-6) of antithyroid therapy, and in matched normal controls (black bar). Values are mean ±SEM. P values are those between baseline data of patients and controls.
Metabolic relations between variables
Within the patients, levels of ALS (Rho= -0.36, p=0.049) and IGF-1 (Rho= -0.66, p=0.001) demonstrated significant negative correlations with free T3. However, only IGF-1 concentration demonstrated a significant negative correlation with free T4 (Rho= -0.56, p=0.005). ALS and IGF-1 were not associated with TSH, nor with age, BMI, fasting glucose, insulin, intact proinsulin, or with IR (Figure 3).
The results of the present study confirm that hyperthyroid status affects IGF axis and is associated with discrete and reversible changes in ALS and IGF-1. Both were significantly decreased in untreated hyperthyroidism. The degree of reductions in ALS and IGF-1 were inversely correlated with the severity of hyperthyroidism as their levels increased significantly to similar concentrations of controls after antithyroid medical treatment and achievement of euthyroidism. Previous studies have reported several alterations of the IGF-1 in hyperthyroidism. Serum concentrations of IGF-1 have been reported to be normal18,19 or high18,20-22 in untreated hyperthyroidism. Moreover, control of the thyroid function either decreases.20,22 or does not modify18 serum IGF-I levels. Data on the changes of ALS with altered thyroid status were limited. In a small study of 24 thyrotoxic females, small reductions in ALS levels were observed with ATD therapy,23 whereas others reported low ALS levels in untreated hypothyroid subjects that have increased after thyroxin treatment.24 To our best of knowledge, this study is the first controlled description of combined alterations in ALS and IGF-1 in hyperthyroid patients and controls where the patients were studied before and after medical treatment. We found decreased levels of ALS and IGF-1 in untreated hyperthyroid subjects compared with controls, which were normalized after therapy with anti-thyroid drugs and attainment of normal thyroid status. Differences in the studied sample sizes, differences in population groups, age ranges, and body composition as well as diverse diseases etiology, severity, evolution time of the thyroid hyperfunction, and the duration of dysthyroidism might account for the difference in reported investigations. The mechanism for the finding of decreased ALS and IGF-1 in untreated hyperthyroidism could be partly attributed to the high insulin levels and elevated insulin resistance in hyperthyroidism, a major finding in our study that is well known to negatively alter the secretion of GH/IGF-1/ALS.25 Also we cannot exclude the possibility of an increased metabolic clearance rate effect persue, similar to what has been observed with other hormones such as prolactin and GH that show decreased serum concentrations in hyperthyroid patients despite increased production rates.26 Furthermore, the finding of significant negative correlations of ALS and IGF-1 with free thyroid hormones is another documentation of their negative link with overactive thyroid status. So it is possible that these changes are as a result of direct negative actions of thyroid hormones as the alterations in ALS and IGF have been shown to be reversible upon induction of an euthyroid state.27,28
The protein Acid-Labile Subunit (ALS) plays an important role in prolonging the half-life of insulin like growth factor-I (IGF-I) and its principal binding protein IGF binding protein-3 (IGFBP-3) in the circulation. Low ALS and IGF-1 during childhood are associated with growth retardation and a mild insulin resistance. In adulthood, however, less is known about the clinical presentation of ALS deficiency. Furthermore, low IGF-I levels in healthy middle-aged populations have been associated with increased risk of ischemic heart disease29-31 and increased risk of developing congestive heart failure.32 Additionally, once heart failure is clinically present, it has been shown that low IGFI levels are associated with a worse prognosis.33,34 These findings suggest that low IGF-I status in adulthood may have implications for the development of premature atherosclerosis or progression of cardiovascular disease.35 Although epidemiologic studies which have assessed the association between IGF-I levels and cardiovascular disease are inconsistent, it is hypothetically, therefore, possible to partially consider a negative role of the sustained low levels of IGF-1 and ALS in the cardiovascular adverse manifestations commonly seen in untreated hyperthyroidism. The observed concomitant elevations in insulin and intact proinsulin levels suggest that the secretary function of beta cells of the pancreas is supraphysiologic in patients with hyperthyroidism. Despite this finding, levels of glucose were also raised, a reflection of insulin insensitivity that was confirmed by elevated HOMA-IR in hyperthyroid patients compared with controls. The fact that all these changes were reversed after attainment of euthyroidism indicates that the effects on pancreatic cells and insulin are transient and dependent on thyroid status.36 Important implications from this study are to be considered. Because hyperthyroidism is shown to be associated with transient and reversible alterations in ALS and IGF-1, several issues should be dealt with critically in untreated subjects. Firstly, in untreated hyperthyroidism, the diagnosis of growth hormone deficiency based solely on low IGF-1 should be considered cautiously because IGF-1 level may increase after treatment of hyperthyroidism and achievement of euthyroidism. Secondly in patients with GH deficiency and hyperthyroidism, physicians need to anticipate the possible hyperthyroid lowering effects of IGF-1 during GH therapy and should be aware in adjustment of dose of treatment accordingly.
Hyperthyroidism is a hyper catabolic state and ofcourse will be a growth blunting disease. It is therefore possible that until euthyroid status is resumed any other factors for growth impairment are considered less influential than hyperthyroid status.
This prospectively designed controlled study describes certain alterations in the circulating levels of IGF-1 and ALS in patients with hyperthyroidism before and after treatment. ALS and IGF-1 were both low in untreated hyperthyroidism and were inversely related to at least one of the thyroid hormones (free T3 and free T4). They increased to similar levels of control subjects after attainment of euthyroidism. It would be possible to consider reduction in levels of ALS and IGF-1 as potential supportive markers of altered thyroid status, namely hyperthyroidism and normalization of their levels would complement the thyroid function test to indicate disease remission during follow up with definitive therapy.
We thank all the participants in this study. We thank Dr. Hanan Makhlouf from the Endocrine division of Mubarak Al-Kabeer Hospital who played a role in recruitment of some patients and Dr. Mona Al-Zaid from the hormone laboratory of Mubarak Al-Kabeer who measured thyroid function for the subjects.
The author declares there is no conflict of interest.

©2017 Al-Shoumer, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.