eISSN: 2473-0815


Case Report Volume 2 Issue 1
1Department of Medical Pathology, National University of Asuncion, Paraguay
2Department of Endocrinology, Federal University of Sao Paulo, Brazil
3Laboratory of Molecular Medicine, Federal University of Sao Paulo, Brazil
4Social Security Institute, IPS, Paraguay 5University of Buffalo, USA
5University of Buffalo, USA
6Nuclear Center for Diagnosis and Treatment and Endocrine Center and Diagnostic Imaging, CEDIN, Brazil
Correspondence: Jorge Antonio Jara Yorg, Department of Medical Pathology, National University of Asuncion, Director of the Nuclear Center for Diagnosis and Treatment and Endocrine Center and Diagnostic Imaging, CEDIN, Representative and National Coordinator of ICCIDD in Paraguay, Paraguay
Received: March 03, 2015 | Published: April 8, 2015
Citation: Jara YJA, Maciel MBR, Cleber, et al. Case report of a thyroid medullary carcinoma and hyperparathyroidism in patient with ret proto oncogene positive. Endocrinol Metab Int J. 2015;2(1):40-47. DOI: 10.15406/emij.2015.02.00009
Medullary thyroid cancer is a rare malignant neoplasm of the epithelium that derives from the C cells (parafollicular) and occurs spontaneously or as part of multiple endocrine neoplasia syndrome. The diagnosis is made by histopathology and clinical history. Might be familiar due to genetic mutation which can be identified by the genetic testing ret oncogene. The main marker is Calcitonin in blood. Prophylactic surgery in children and youth is indicated in MEN 2 (Multiple Endocrine Neoplasia) .In this study we present a case of Ca Medullary Thyroid diagnosed post operatively and where the family including sons, brothers and nephews were studied for the ret oncogene mutation. Results showed that 2 of the children were positive and due to the diagnosis were operated. One showed medullary Thyroid Ca associated hyperparathyroidism and the other was negative. Ret proto oncogene mutation studies were performed in patients with this pathology in Paraguay for the first time. The association of Thyroid Medullary carcinoma and hyperparathyroidism is rare and both were resolved with surgery and ablative therapy with 131I-MIBG
Keywords: medullary thyroid carcinoma, RET, proto oncogene 131I MIBG, total, thyroidectomy, hyperparathyroidism
MEN, multiple endocrine neoplasia; FMTC, familial form of medullary thyroid carcinoma; MIBG, meta iodo benzyl guanidine; CEDIN, center of nuclear diagnostic and treatment
Medullary carcinoma corresponds to a C-cell neoplasm and constitutes 4% to 5% of all cancers affecting the thyroid gland.1–3 This condition does not originate in the follicular cells of the thyroid but parafollicular cells and represents a malignant transformation of C cells derived from the neuroectoderm. These C cells and their malignant counterparts secrete the hormone calcitonin which is important for the diagnosis and postoperative follow in pursuit of irradiation.4 Approximately 75% are sporadic and are not hereditary, yet 25% are hereditary and can be MEN (multiple endocrine neoplasia) type which is associated with different mutations of the proto-oncogene RET4, and can be of three types MEN 2A, MEN 2B, and familial form of medullary thyroid carcinoma (FMTC).5–7
MEN type 2 is an autosomal dominant disorder characterized by bilateral or multiple pheochromocytoma and parathyroid hyperplasia. There are 19 mutations of exon 10 and 11 of the extracellular domain of the RET protein. Hypercalcemia is a late manifestation and indicates hyperparathyroidism. The hyperparathyroid glands may reveal cell hyperplasia or only hyper cellularity.
Pheochromocytoma develops in approximately 50% of patients with MEN2A and hyperparathyroidism develops in approximately 10% and 30%.8 The sporadic form usually presents in the fourth decade of life as a thyroid mass and is diagnosed by ultrasound, cytology obtained with the needle aspiration biopsy (FNA) with ultrasound and histochemical study of the dosage of calcitonin in preoperative serum is highly sensitive and specific for medullary thyroid carcinoma and reveals residual disease and long–term monitoring of patients.
Calcitonin is a 32 amino acid polypeptide hormone secreted by the parafollicular cells, which acts to depress serum calcium levels by inhibiting osteoclastic activity and increasing calcium renal, also increases intestinal secretion of water and electrolytes. Basal calcitonin levels correlates with tumor burden.9 The increase of calcitonin levels in patients with widespread metastatic disease have been associated with chronic and severe diarrhea. Calcitonin elevation generally occurs in the C-cell hyperplasia and all forms of medullary thyroid carcinoma. However, patients with hyperplasia of C cells or early medullary carcinoma may have normal levels of basal calcitonin. Often diagnosed by pathology at surgical removal, around 60-80% of these tumors have lymph node metastases.10–11 Microscopically, both forms sporadic and hereditary of Medullary Thyroid Carcinoma are similar. An important distinguishing feature includes C-cell hyperplasia and multifocality in inherited forms. The amyloid tissue is present in 80% of spinal tumors, is believed to represent degenerate gene products calcitonin.12–14
Hyperthyroidism may be associated with this condition, whereby the dosage of thyroid hormones must be made compulsorily. The RET proto oncogene test is a genetic study to assess whether the tumor is hereditary. However, in the 3% to 5% of patients with MEN 2a and in 15% of FMTC patients, cannot identify mutations of the RET oncogene.10 In surgical studies, mutational RET oncogene test has not been associated with false negative or false positive results.16 The RET oncogene test has been pre-operatively recommended in all patients with Medullary carcinoma.17–19
Eng and colleagues20 estimated that a patient without a known RET mutation Germ line has a 0.5% chance of MEN 2A and 3% of FMTC. The 10-year survival of patients with tumors confined to the thyroid gland tumors is 95.6%, those with metastases to regional lymph nodes is 75.5% and those with distant metastases is only 40%.21 Primary hyperparathyroidism has been reported between 10-25% of patients with MEN 2A.22,23
The ablative treatment of post surgical debris with 131I MIBG (metaiodobenzylguanidine) is also performed for remnant ablation in patients undergoing surgery.24
Genetic Genetic study of oncogene RET was made to a male patient 65 years of age operated in the Central Hospital of the Institute of Social Welfare of Asunción (Hospital of Social Security), Paraguay with a history of hyperthyroidism treated in 2006 at the Center of Nuclear Diagnostic and Treatment (CEDIN) at a dose of 15 mCi (555MBq) 131 I orally. Later he was medicated with 200 mcg / day of L-thyroxine sodium replacement orally.
Due to the growth of a thyroid nodule in the right lobe of 3 mm in diameter, one in isthmus and another in the left lobe ultrasound six years later, in 2012 the patient underwent a total thyroidectomy. One of the nodules was Medullary Thyroid Carcinoma post operatively diagnosed by pathology (Figure 1).
The study of the RET Proto oncogene was held at the Hospital de Clinicas, University of Sao Paulo, Brazil giving a positive result, so we decided to study the 18 immediate relatives (Figure 2).
In the patient diagnosed preoperatively Calcitonin was 77 pg/ml (RR: 13.5-39.5 pg/ml) and post total thyroidectomy with lymph node dissection was reduced to 1 pg/ml (RR: 13.5-39.5 pg/ml) The values thyroid hormone were less than 0,01uUI/ml (0,35-4.94) TSH, T3: 125 ng/dl (58-159), FT4: 1.59 ng/dl (0,70-1.48) TPOAC: <a1UI /ml (VN <a 5.61UI/ml), TGAC <2 IU/ml (less than 4.11 UI7ml VN), Thyroglobulin 2.12 (ng/ml). The preoperative PTH was 315.1 pg/ml (RR: 15.0-65.0 pg / ml) which was reduced in the postoperative period to 51.5 pg/ml. (RR: 15.0 - 65.0 pg/ml). The CEA (carcinoembryonic antigen pre-operative was 69.0 ng/ml (VN non smoking up to 3 ng/ml and smoking up to 5 ng/ml) and postoperative was 3.2 ng / ml. The antigen CA 19-9 value was 51.4 U/ml (less than 27 U/ml VN). Treatment with 3 mCi of 131I MIBG (111 mBq) was performed three weeks after surgery unmanaged thyroid hormone replacement reduced its Calcitonin pg 3 pg 17 (RR: 13.5-39.5 pg/ml.). The thyroid and Parathyroid Scintigraphic images were taken using a gamma camera Spect (Mediso) in the pinhole collimator CEDIN SRL (Figure 3), (Figure 4 & Figure 5).



Of the 18 immediate family (6 kids, 9 siblings and 3 nephews, sons of a deceased brother) in whom the genetic study of the RET oncogene was performed at the Hospital de Clinicas in Sao Paulo, two sons of the patient, the first male (33) and one female (18) were positive and led to surgery (total thyroidectomy):
We receive for diagnosis by freezing without fixative corresponding to the total thyroidectomy surgical piece. It is fixed in buffered formalin, with long wire marker pole top right and left superior short thread pole. 3.7 grams total weight of right lobe with 2.5x2x0.5 cm with isthmus 2x1 cm and left lobe 3x1x0.6 cm. External surface greyish, lobed, covered by a fibrous capsule. When cut three nodular formations are observed. A) A level of the lower pole of the right lobe nodular, solid, firm yellowish lesion of 0.4x0.3x0.3 cm .B ) At the level of the isthmus another brownish lesion of 0.4 cm and C) At the level of the left lobe a nodule 0.7 cm Rest of thyroid parenchyma is reddish brown in appearance preserved. Total inclusion of the material in 9 capsules.
A piece of tissue is received irregularly shaped measuring 9 x 3,5 x 0,3 cm is received . Brownish yellow outer surface .When cut woven elastic solid. Partial inclusion of the material on 5 capsules. (Dr. Camila Montoya)
Medullary thyroid carcinoma metacentric .A focus of 0.4 cm was observed in the right lobe. Another 0.4 cm in the isthmus and another 0.7 cm in the left lobe. Neither infiltrates the thyroid capsule extends to brownish fibro fatty tissue. Rest of the parenchyma shows no histopathological changes.
1Hyperplastic lymph nodes (16) without evidence of metastasis. Dr. Enrique Ayala, Pathological Anatomy IPS, Professional registration HC 4713.
Of the 18 immediate family (6 kids, 9 siblings and 3 nephews, sons of a deceased brother) in whom the genetic study of the RET oncogene was performed at the Hospital de Clinicas in Sao Paulo, two sons of the patient, the first male (33) and one female (18) were positive and led to surgery (total thyroidectomy). (Figure 6), (Figure 7)
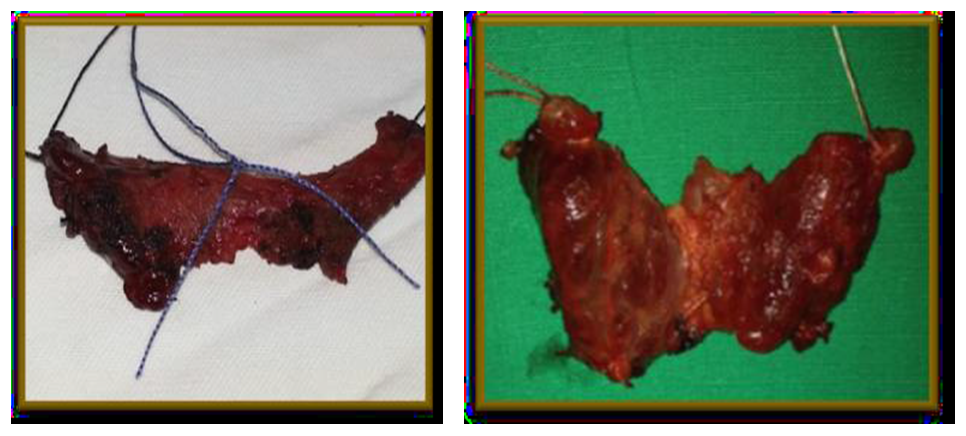
Figure 7 Surgical specimens of both patients.
The 33 year old son presented two nodules of Medullary Thyroid Carcinoma post operatively diagnosed by pathology and was treated with 131 I MIBG.
The 33 year old son presented two nodules of Medullary Thyroid Carcinoma post operatively diagnosed by pathology and was treated with 131 I MIBG. (Figure 8), (Figure 9)
|
Father |
Son |
Daughter |
|---|---|---|---|
Age |
65 |
33 |
18 |
Thyroid Ultrasound |
Nodular Hyperthyroidism |
Nodular Hyperthyroidism |
Normal |
Diagnosis |
Multinodular goiter |
Multinodular goiter |
Diffuse goiter |
Calcitonine pre operatory |
75 pg/ml |
25 pg/dl |
20 pg/dl |
Calcitonine Post Operatory |
17 pg/ml |
2.0 pg/ml |
4.4pg/ml |
Post 131I MIBG |
3.0 pg/ml |
0.5 pg/ml |
2,5pg/ml |
PTH pre operatory |
315.1 pg/ml |
163pg/ml |
0.5 pg/ml |
PTH post operatory |
51.5pg/ml |
48.5pg/ml |
0.2 pg/ml |
TSH post operatory |
22 µUI/mL |
15.66 µUI/mL |
17 µUI/mL |
TSH post LT4 |
0,36 µUI/mL |
0,12 µUI/mL |
2,13 µUI/mL |
Ret Proto Oncogene + |
+ |
+ |
+ |
Table 1 Patients with positive RET Proto Oncogene test
Calcitonin, TSH and PTH decreased after surgery and treatment with 131I MIBG
Name |
Sex |
Age |
RET Proto Oncogene |
BRP |
M |
65 |
+ |
MCRP |
F |
68 |
- |
MBRP |
F |
66 |
- |
MRRP |
F |
60 |
- |
DRP |
M |
55 |
- |
CMLRP |
F |
51 |
- |
VRP |
M |
53 |
- |
NRP |
M |
49 |
- |
MVRP |
F |
56 |
- |
JMRP |
M |
46 |
- |
MSF |
F |
18 |
+ |
RR |
M |
33 |
+ |
BRS |
F |
30 |
- |
ERS |
M |
28 |
- |
DRS |
F |
31 |
- |
BR |
M |
16 |
- |
BR |
F |
15 |
- |
SF |
M |
20 |
- |
MR |
M |
31 |
|
# Feminine |
9 |
|
|
# Masculine |
10 |
|
|
Total |
19 |
|
|
Age range |
39.55 |
|
|
Table 2 Number of relatives studied with RET Proto Oncogene
MEN |
|||
|---|---|---|---|
Syndrome |
Cases with known RET mutations |
Exon |
Codons |
MEN 2A |
97% |
10 |
609,611,618,620 |
11 |
630,634 |
||
13 |
768,790 |
||
MEN 2B |
95% |
15 |
883 |
16 |
918,922 |
||
FMTC |
86% |
10 |
609,611,618,620 |
11 |
630,634 |
||
13 |
768,790,791 |
||
14 |
804 |
||
15 |
891 |
||
Table 3 This table indicates the possible relations between the syndromic mutations, its percentage and the specific location of the mutations
Variable |
Number of patients |
3 |
Age average (years) |
38,66 ± 1 |
|
Gender (male/female) |
2/1 |
|
Clinical presentation |
Cervical volume increase |
2 |
Nodules |
2 |
|
Findings |
2 |
|
Time of evolution (mean in years) |
1,9 ± 2,6 |
|
Preparatory Diagnosis |
2 |
|
Post operatory diagnosis |
2 |
|
Type of MTC |
Sporadic |
0 |
Familiar |
3 |
|
NEM 2a |
0 |
|
NEM 2b |
3 |
|
Surgery: |
3 |
|
TT |
10 |
|
TTA |
10 |
|
Adjuvant treatment |
QMT |
0 |
131I MIBG |
2 |
|
Histological characteristics |
Average tumor size (cm ) |
3,5 |
Bilateralism |
2 |
|
Capsular commitment |
1 |
|
Lymphatic metastasis |
1 |
Table 4 Clinical and histological characteristics of patients with positive RET proto Oncogene
Surgical treatment consisted of total thyroidectomy with bilateral lymphadenectomy in three patients with positive RET Oncogene. The father had three nodules with Medullary carcinoma, one in the right lobe, one in isthmus and another in the left lobe, with 16 benign lymph node metastases without evidence of pathology. Values presurgical Calcitonin was: 25 p /dl (RR: 13.5-39.5) and postoperative less than 2.0pg / ml (less than 8.4 RR) PTH was 85 pg/ml and 163 presurgical pg/ml immediately post surgically (UGRR 13.5-39.5 pg/ml). Two weeks after surgery dropped to 48.5 pg/ml and after treatment with 131I MIBG was 2 pg/ml. With the result of Oncogene positive RET and due to the presence of a nodule of 4 mm in diameter in the right lobe was operated in the IPS, surgery confirmed the finding of tumor 4 mm in diameter in the right lobe and smaller in the isthmus and the left lobe.
The outcome and survival of patients with Medullary Carcinoma are directly related to the surgical removal. The three patients who underwent total thyroidectomy and lymph node dissection showed no metastases in regional lymph nodes. Central prophylactic dissection was performed in three patients who had positive RET Oncogene. Two of them men, who were hyperthyroid and treated first with I131, after 5 years developed thyroid nodules and elevated values of PTH with an association with hyperparathyroidism. This disease has a low recurrence in operated patients.20 Both patients underwent surgery and subsequently treated with therapeutic doses 131I MIBG achieving normal calcitonin levels, despite limited results reported in the literature using the 131I MIBG and somatostatin analogues.21–22
Early screening should be seek in the potentially affected relatives for diagnosis, treatment and better prognosis of MEN 2 once detected in one of the family members. Biochemical testing should be performed in those with negative RET oncogene.
The 18 relatives related to the first patient with CMT oncogene RET + were studied in the Hospital of Clinics in Sao Paulo with the help of the head of Endocrinology Prof. Dr Rui Maciel and members of his department at no cost to patients. In hereditary disease, the type has prognostic importance. Adjuvant therapy, including radiotherapy and chemotherapy are often beneficial when the patient has obstructive symptoms or potential risk of cancer growth.
Pathologically, in the presence of bilateral thyroid disease the hereditary form of the disease should be suspected. Because somatic RET oncogene mutations occur frequently in sporadic disease genetic analysis of oncogene should be performed.
The RET Proto Oncogene is an important genetic study to rule out familiar Medullary carcinoma, whereby all patients diagnosed with this condition should be genetically studied with RET Oncogene, which is performed only in specialized centers. Importantly, the immediate and next of kin positive patient should be studied due to the probability of developing the disease and for surgical therapy. Radical surgery of the thyroid with lymphadenectomy associated with 131I MIBG enable rapid decrease in blood levels of calcitonin and PTH.
Prof. Dr. Rui Maciel, Head of Department of Endocrinology at the Federal University of Sao Paulo, Brazil.
Dr M. B. Cleber Camacho, Deputy Head of the Department of Endocrinology at the Federal University of Sao Paulo, Dr. Kunii Sizue, Biomedical responsible for conducting the RET Proto Oncogene the UFSP for having collaborated and performed studies free of charge to all patients. Prof. Dr. Francisco Perrotta, in charge of the surgeries of the patients.
In this paper there is no conflict of interest. The funding of this study were provided by the CEDIN, the Social Security Institute for surgery of its insured and the Federal University of Sao Paulo for studies of RET Oncogene.

©2015 Jara, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.