eISSN: 2473-0815


Case Report Volume 12 Issue 4
Ministry of Higher Education, Iraq
Correspondence: Saadi JS AlJadir, Ministry of Higher Education, Iraq
Received: September 25, 2024 | Published: October 14, 2024
Citation: Saadi JS AlJadir. Case Report: Headaches and Hormonal Disruptions. Endocrinol Metab Int J. 2024;12(4):101-107 DOI: 10.15406/emij.2024.12.00355
Craniopharyngiomas are rare, relatively benign, and slow-growing tumors that arise near the pituitary gland and hypothalamus and in the vicinity of the center of the brain. Therefore, the tumor is usually discovered to be big enough to cause presenting symptoms, including headaches, vomiting, and visual problems. Variable hormone deficiencies that lead to poor growth or absent puberty might also be found in children and adolescents, while in adults, such as thyroid underactivity, adrenal crises, gonadotropin deficiency, and diabetes insipidus. Although patients with craniopharyngioma have an excellent survival rate, this tumor’s tendency to adhere to important parts of the brain renders complete resection unattainable and, therefore, leads invariably to tumor recurrence. Thus, patients with this type of tumor must undergo regular, appropriate imaging, by MRI or CT scan. Craniopharyngiomas develop in around 0.5 to 2 per million individuals each year and the neoplasm has a commonly dual peak of age distribution, with the highest rate in 5 to 14 years and another wave in adults between 50 and 74 years of age accounting for 1% to 3% of all brain tumors. There are no gender differences, but racial differences are noted.1 The case reported here was a middle-aged lady who had presented with the common presenting symptom, which is a headache, associated with vomiting and variable hormonal disturbances. Treatment options include surgery, radiation, or both; hence, our patient had been subsequently submitted to neurosurgical intervention with preoperative and postoperative hormonal assessment and management. More than one specialty is required for the management of this neoplasm; a team of endocrinologists, ophthalmologists, neurosurgeons, and psychologists might be needed to improve the quality of the patient’s life.
CP; Craniopharyngioma, MRI; Magnetic Resonance imaging, MRA; Magnetic Resonance Angiography, CT; CAT-Scan, GH; Growth Hormone, DHEAS; Dehydroepiandrosterone sulphate, TSH; Thyroid Stimulating Hormone
Craniopharyngiomas are rare, benign tumors of the central nervous system. They are epithelial tumors that invariably arise in the suprasellar area of the brain, ascending to involve the hypothalamus, optic chiasm, cranial nerves, third ventricle, and major blood vessels (Figure 1a&1b).
It might present with a wide variety of symptoms, from headaches, nausea, and vomiting to visual and endocrine disturbances. It creates appreciable challenges for the treating physicians. Curative surgeries are generally unattainable and complicated due to their location and infiltration into the vicinity of surrounding structures. Furthermore, the quality of life is generally jeopardized due to the development of multiple complications: panhypopituitarism, visual problems, obesity, and mental disorders (Figure 1a). 2–4
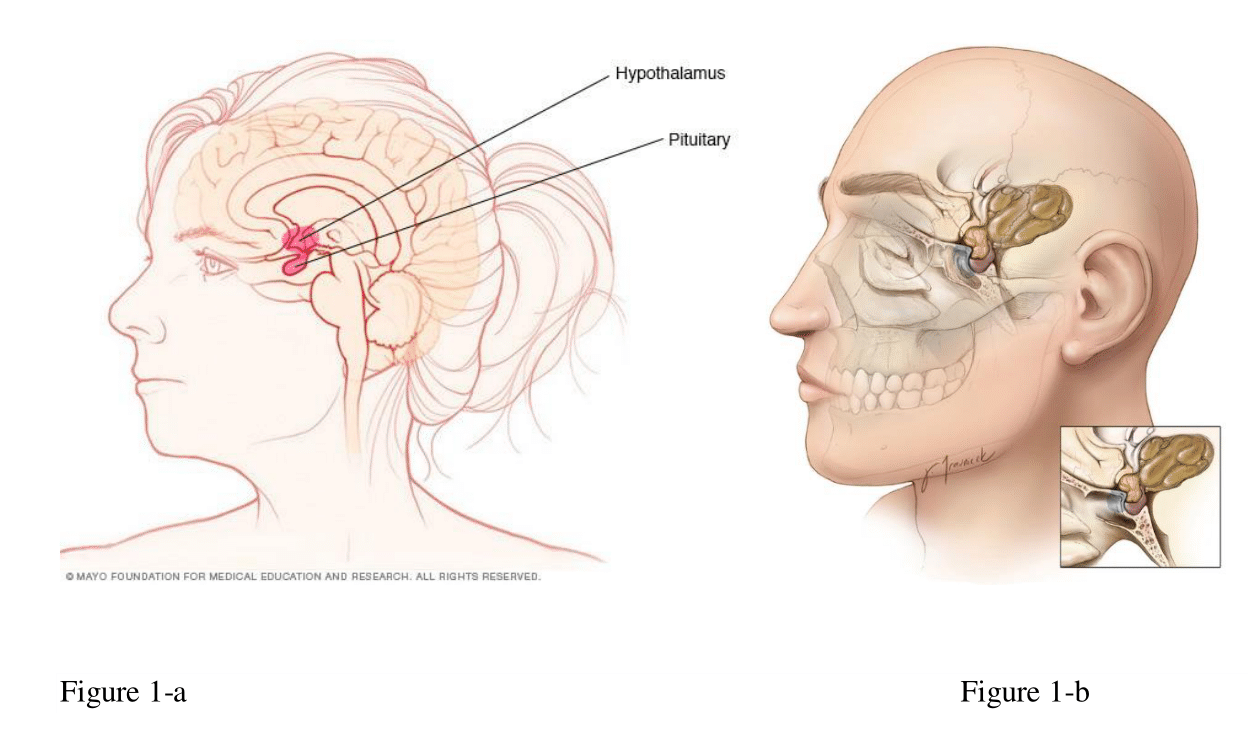
Figure 1 A craniopharyngioma (yellow brown) sitting at the base of the skull on top of the pituitary gland. A characteristic cystic component extends toward the middle of the brain. (Courtesy of Aaron Cohn-gadol.com and neurosurgicalatlas.com).
WHO has considered craniopharyngioma a grade I neoplasm. There are two histopathological varieties: Adamantinomatous and papillary. The first one most commonly occurs in children, while the second is more common in adults. Adamantinomatous craniopharyngiomas arise from cells from an embryologic structure called the craniopharyngeal duct (Rathke’s pouch). They can be solid or cystic structures filled with dark brown to black fluid and are often calcified. Papillary craniopharyngiomas are rarer and arise from cells of the anterior part of the pituitary gland. They are generally well-defined and can also be solid or cystic, although they are filled with yellow and viscous fluid. They rarely calcify. However, the clinical manifestations of both types are largely similar. As craniopharyngiomas are rare tumors, they are characterized by atypical and non-specific symptoms; usually, they are diagnosed years after the appearance of symptoms. An early diagnosis is favorable, as it can greatly improve the quality of life of affected individuals and reduce the risk of permanent hormonal derangements and long-lasting complications. Craniopharyngiomas currently do not have either a well-documented etiology or associated risk factors. Researchers believe that craniopharyngiomas might arise from the abnormal development of cells that form parts of the pituitary gland in the growing fetus. Moreover, few cases had been reported running in families5,6
Craniopharyngioma is one of the most challenging neoplasms and was first described by Friedrich Albert von Zenker in 1857. Jakob Erdheim (Austrian pathologist) was the first to precisely describe the histopathological characteristics. The current name had been used in 1932 by Harvey Cushing. Babinski described the clinical presentation of children with sexual infantilism and dystrophic adiposity. A. E. Halstead of Chicago performed the first successful surgical resection of the tumor on July 21st, 1909. Harvey Cushing entertained the transsphenoidal approach for most pituitary operations but preferred the transcranial approach for craniopharyngiomas. A great improvement in successful surgical management was achieved after discovering antibiotics, corticosteroids, and microscopy.
Over time and with technological advances, transcranial and transsphenoidal routes were entertained according to tumor features. The introduction of more adjuvant modalities, like stereotactic radiosurgery, radioisotope brachytherapy, and intracystic chemotherapy (e.g., bleomycin, interferon α), has improved tumor resection, decreased the chance of recurrence, and reduced complications.7
Craniopharyngioma has an incidence of 0.5 to 2 cases per million persons per year. Generally, it is considered primarily a childhood neoplasm; however, it can be detected at any age. And there is an appreciable number of cases that are diagnosed in adults. It has two distribution waves: 5–14 and the other one between 50 and 74 years of age. There is no gender, ethnic, or geographical distribution difference in the incidence of the neoplasm. Craniopharyngioma has long been recognized as having a high recurrence, and in some series, it has been estimated to be as much as 50%. Despite having good survival rates, however, it carries similar rates of morbidity throughout most of the patient’s series. 8,9
Craniopharyngiomas are recognized as having insidious onset, and this critical reason could be attributed to the delay in the early detection and diagnosis, usually made after the eruption of pressure symptoms. The pressure symptoms are exclusively attributed to 1) CSF flow obstruction resulting in raised intracranial pressure or 2) direct pressure thereby resulting in damage to the pituitary gland and adjacent optic apparatus in the vicinity. There is often 1-2 years from the onset of symptoms and diagnosis.2–4
Symptoms are varied; they might be few or clusters, and the following are the recognized symptoms in many patients:
There are two histological varieties of craniopharyngiomas: a) the adamantinomatous tumors, the type that usually occurs in children, whereby the structures mimic enamel and therefore form neoplasms in the oropharynx and b) the squamous papillary form, mostly seen in adults. Therefore, according to the histology of the neoplasm, the characteristic location of these tumors in the sellar and parasellar regions will lead us to limit the etiology into two theories.
This suggests that the adamantinomatous type arises from epithelial remnants of the craniopharyngeal duct or Rathke's pouch. The duct and pouch were derived from the stomodeum (the primitive oral cavity), which forms preliminary teeth and related structures.
The squamous papillary type, which is mainly seen in adults, might occur because of metaplasia of squamous epithelial cells, which are remnants of the buccal mucosa of the stomodeum. These remnants can undergo metaplasia and form squamous cell nests, which might undergo more proliferation and later lead to this kind of neoplasm. Somatic mutation in BRAF has been associated with this neoplasm. BRAF activates the mitogen-activated protein kinase (MAPK) pathways, which are usually amplified in the neoplastic process. Another presumptive mechanism in etiology is the involvement of beta-catenin, which is known as a transcriptional activator of the Wnt signaling pathway, which plays a critical role in embryogenesis and neoplastic potential.5,6,10
The diagnosis of a patient with craniopharyngioma is based exclusively on clinical (neurologic and endocrine) and radiological findings by appropriate imaging techniques, and ultimately the postoperative specimen by histopathological examination.11–14
The well-recognized topography of a craniopharyngioma is of either seller or para-seller partially solid mass, cystic, or calcified mass (Figure 2).
These tumors are in:
The suprasellar-located tumors are considered further according to their relation to adjacent structures like; the third ventricle or the optic chiasm.
A CT scan can show calcification better than other imaging, while MRI can appreciably delineate the extent of the tumor and its relation to the hypothalamus and adjacent structures, therefore, it is sound imaging preoperatively; moreover, MRA can differentiate the tumor mass from vascular malformations like aneurysm (Figure 2).15,16
Of the utmost importance is the hormonal assessment and consequently should manage the specific endocrine abnormality in pre-operative and post-operative stages. These include the hormones that comprise the hypothalamic-pituitary axis, which are growth hormone, IGF-1, thyroid stimulating hormone, Free T4, luteinizing, and follicle-stimulating hormone, testosterone (males), estradiol (females), prolactin, Fasting serum cortisol, ACTH, serum sodium measurement of serum and urine specific gravity and osmolality (if diabetes insipidus is suspected). Of note, dynamic testing by synacthen stimulation might be done if morning cortisol is indeterminate.17
Clinical presentation
MRI of the pituitary 05.02 (Figure 4).
Conclusion
The imaging findings are those of Craniopharyngioma (Figure 4).
Blood Tests
Blood Chemistry
Hormonal Profile
Hormonal Profile
Morning (6.2-19.4)
Evening (2.3-11.9)
DHEA-S 2.640 µmol/L; low.
Hormone replacement
SKMC (Tertiary Centre) Report:
MRI Diagnosis
Conclusion: findings are consistent with post-operative changes? Ischemia of small vessel disease
Hormonal Assays
Management: Medical
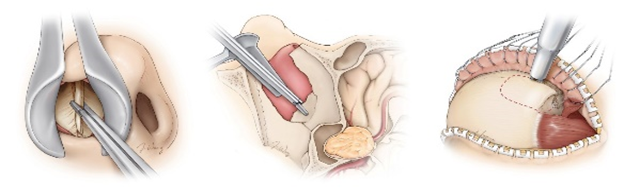
Figure 6 Trans-nasal approach (left & Middle), trans-cranial (Right) for resection of CP.courtesy of Aaron Cohn-gadol.com and neurosurgicalatlas.com.
Concept of the Diagnosis
Histopathology
Conclusion: The impression was that of purely hormonal pituitary adenoma with focal necrosis and apoplexy.
Management Strategy
The patient was referred to us and received in the Diabetes and Endocrine Clinic, for scheduled evaluation and follow-up:
Differential diagnosis
Congenital anomalies: Arachnoid cyst and Rathke's cleft cyst.
Brain Neoplasms: Pituitary tumor, metastasis, meningioma, epidermoid tumor, optic glioma, hypothalamic hamartoma, and teratoma
Inflammatory Lesions: Eosinophilic granuloma, Lymphocytic hypophysitis, Granuloma (sarcoidosis, syphilis, tuberculosis, etc.)
Vascular anomalies: Aneurysm of the internal carotid or its branches, arterio-venous malformation.2,3
The clinical significance of the patient's presentation will determine the type of surgical intervention. In the presence of acute deterioration of vision and progressive raised intracranial pressure or hydrocephalus, therefore immediate action is decompression as much as possible, and choosing the appropriate intervention.
The clinical significance of the patient's presentation will determine the type of surgical intervention. In the presence of acute deterioration of vision and progressive raised intracranial pressure or hydrocephalus, therefore immediate action is decompression as much as possible, and choosing the appropriate intervention.
Before definitive surgical action has been taken, the topography of the neoplasm to the hypothalamus should be considered to determine whether drastic debulking or partial tumor resection is needed. If the tumor extends or involves the hypothalamus, total resection with damage to the hypothalamus will be hazardous and will lead to variable neuropsychological derangements. Some neurosurgeons may sacrifice the pituitary gland by debulking the tumor to avoid future inevitable recurrence (as in our case), cases of residual tumors whereby radiotherapy has been entertained, yet there is a significant number of recurrences of the disease; therefore, drastic surgery was the preferred intervention undertaken. Moreover, tumor removal, especially in the past 2 decades, as the new modalities of treatments had not proven efficacy yet! Interestingly, some reports stated that thalamic involvement in adult patients is quite less than in children. The residual neoplasm that invariably remained unrespectable can be managed by adjuvant radiotherapy; external beam, stereotactic (gamma knife), and proton beam radiotherapies have been tried in some centers. Other procedures might be applied, like an AV shunt for hydrocephalus or a catheter inserted for repeated aspirations in purely solitary cystic lesions.18–20
The commonest modalities of treatment
Radiation by a variety of modalities has been used for those patients with incomplete resection or might be for preventing relapse. Radiation therapy includes conventional external radiotherapy, proton beam therapy, stereotactic radiotherapy, radiosurgery, and brachytherapy. Many reports have proven decreased mortality with slightly reduced morbidity following radiation therapy. In well-experienced centers, radiotherapy has not been advocated for disease recurrence; therefore, they are considered the adjuvant treatment for neurosurgery.
This modality of treatment is used exclusively for cystic neoplasm. Intracystic radiotherapy (Yttrium-90 or Phosphorus-32) or chemotherapy, including bleomycin and interferon α, have been used with variable reports of success. This modality of treatment is aimed at causing tumor shrinkage and leading to sclerosis and fibrosis. The negative drawback of this modality has been many reported incidents of severe neurotoxicity that are attributed to installed substances.21–23
In most series, there is generally improvement in the visual deterioration if present preoperatively; a periodic ophthalmological check-up is recommended; on the other hand, the endocrine abnormalities are usually permanent and might be amplified after surgery. Obesity might be present in an appreciable number of patients, whereby most patients require two or more anterior pituitary hormone replacements, and some might suffer from transient or life-long diabetes insipidus; therefore, most patients should undergo lifelong endocrine assessment and follow-up.25
The patient had been on replacement therapy for anterior pituitary hormones (GH, Thyroid, and Cortisol) for the management of diabetes, hypertension, and dyslipidemia. She was doing fine with no evidence of recurrence for 7 years; later, she had been suffering from chronic kidney disease (CKD); however, she was still on conservative treatment.
We had treated a middle-aged woman who had been suffering from repeated bouts of diffused headaches, which were becoming increasingly severe and narrowing in duration. These distinctive elements of her complaints had been brought to clinical notice; therefore the patient underwent urgent imaging, which was strengthened by more appropriate imaging methodology, resulting in the final diagnosis of the tumor. The imaging with adequate enhancement revealed CP, the biochemical work-up revealed varied degrees of anterior pituitary hormonal insufficiencies, and the retrospective history revealed menstrual cessation in the early 30s. The patient was soon stabilized on hormonal replacements, and her pre-existing T2 diabetes mellitus and accompanying complications (hypertension, dyslipidemia) were managed.
Because this is an unusual neoplasm, it is prudent to refer to a specialized facility to ensure a favorable prognosis. In the tertiary center, neurosurgical intervention was performed, including gross resection of the neoplasm and the attached pituitary tissues in the vicinity, as well as measures to salvage important structures such as the hypothalamus, optic chiasm, and blood vessels in the domain, and therefore avoiding complications such as CSF rhinorrhea or at any point obstruction to fluid circulation.
The patient tolerated the surgery well, and the postoperative endocrine assessment and coverage resulted in an excellent and uneventful recovery. Adjuvant therapy was an option in unrespectable cases or when radical resection was not feasible; nevertheless, our patient did not require any more adjuvant radiotherapy or other supportive measures.
This case report serves as a valuable reminder of the complexities surrounding craniopharyngiomas, from diagnosis to management. It emphasizes the necessity of early recognition of symptoms, thorough imaging, and a multidisciplinary approach to care. Future research may focus on better understanding the genetic and molecular underpinnings of craniopharyngiomas to identify potential targeted therapies and improve outcomes for affected individuals.
CP is a rare neoplasm with a bi-modal distribution. Early diagnosis and accurate assessment of tumor topography and involvement of neighbouring structures should be considered when dealing with such patients, as this will result in reduced morbidity and mortality, extending survival and increasing quality of life. To achieve these objectives, a multidisciplinary team of endocrinologists, neurosurgeons, ophthalmologists, psychosocial and rehabilitation specialists will collaborate or work in sequence throughout the patient's disease course. Because this neoplasm is uncommon, it is judicious to be treated by an expert team in a specialized center. Lifetime follow-up is normally recommended for most patients, and our patient was no exception. She had been followed up with a planned scan, and her hormonal status was regularly reviewed and refined as needed.
The authors declare that there are no conflicts of interest.

©2024 Saadi. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.