
Case Report Volume 8 Issue 1
Case report: a virilized girl
Saadi JS AlJadir
Regret for the inconvenience: we are taking measures to prevent fraudulent form submissions by extractors and page crawlers. Please type the correct Captcha word to see email ID.

Ibn Sina Medical Centre, IRAQ
Correspondence: Saadi JS AlJadir, Ibn Sina Medical Centre, PO Box 498 Nassiriya, Thi Qar, IRAQ
Received: September 29, 2019 | Published: January 22, 2020
Citation: AlJadir SJS. Case report: a virilized girl. Endocrinol Metab Int J. 2020;8(1):4-9. DOI: 10.15406/emij.2020.08.00269
Download PDF
Introduction
Giant ovarian tumor has become rare, because of the early detection of adnexal masses with the advent of ultrasound imaging and other modalities. In previous records, the definition of giant ovarian cysts was mentioned as cysts measuring more than 10 cm in diameter in the radiological scan or those cysts that are large enough reaching the umbilicus or above. These tumors occur in about 25% of all benign ovarian neoplasms and 58% of all ovarian serous tumors. Serous tumors are epithelial neoplasms of the ovary account for 60% of all ovarian tumors and 40% of benign tumors. Ovarian cystadenomas are common benign epithelial neoplasms which carry an excellent prognosis, they are commonly seen during the reproductive period and 50% of them occur before the age of 40years. Most of these cysts are benign in nature with the chance of malignancy being only 7%–13% in premenopausal and 8%–45% in postmenopausal women. Serum CA-125 assay is a useful tool that helps to distinguish between benign and malignant ovarian masses, besides the imaging, possibility of malignancy can be confidently excluded. Huge size ovarian serous cystadenoma is rare. In the literature, a few cases of giant ovarian cysts have been mentioned sporadically, majority in elderly patients. We report a 16-year-old girl with a giant left ovarian cyst that secretes androgen, clinically the patient had presented by overt hyperandrogenism. (Virilism), left salpingo-oophorectomy with cystectomy were performed and all specimen has been sent for histopathology. On histopathological examination the cyst was confirmed as benign Serous Cystadenoma of the ovary and biochemically functional (androgen secreting)! Although these neoplasm are rarely hormone-secreting, but our care shows this biochemical and overt clinical features of hyperandrogenism; hirsutism, acne, irregular menstrual periods, male-pattern baldness, loss of female fat distribution, and hoarse voice.1,2
Definition
- Virilization is relatively uncommon; it occurs with extreme hyperandrogenism.
- Virilization is characterized by temporal balding, breast atrophy, androgenic muscle development, clitoral hypertrophy, amenorrhea, deepening of the voice, and extreme hirsutism.3‒9
Case history
- A young girl was presented to our Endocrine Clinic on Sept.2008;
- 15years of age;
- She had been referred as having coarsening and deepening of her voice (male voice!), hirsutism, no periods for last one year; secondary amenorrhea, abnormal scalp hair and abnormal body features that made her family questioned her gender status!
- She had been molested by her classmates as been a boy or rather trans-sexual!, with an enormous worry from her parents!
- She had brought some hormonal tests and ultrasound study.10
Clinical impression
We had examined her with the gynecologist, as patient is socially withdrawn: a teenage girl with obvious signs of virilism; low-pitch voice (coarse &deep!), hirsute, male body habitus, breast atrophy, clitoromegaly (3+ as described by Gynecologist!)
Clinically
- Thyroid of normal size, no LN or abnormal pigmentations.
- Major Systems; no Abnormality.
- No Skeletal defect, or abdominal striae.
- Hair Distribution (Ferriman- Gallway Chart), +21 Hirsutism and Androgen excess…
- Vital signs : BP 123/84mmHg
- PR 74/min
- Weight 44kg, Height 154cm.
Previous records; 15.05.08
- Physician who had referred her with suspicion of being late-onset (Non-classical) CAH…
- 17 (OH) PG 4.4ng/ml (N up to 2.5)!
- ASD 4.3ng/ml ( N 0.7-4.3) Øéé Testosterone 2.68ng/ml (0.060-0.820)
- DHEA-S:7.18µmol/L (up to9.9)
More…
- TFT were WNL
- LH 11.31mIU/ml
- FSH 1.18mIU/ml
- Prolactin 16.65ng/ml
- CEA 2.01ng/ml (less 10)
- CA 125 5.06U/ml (less 35)
Ultrasound study
- Right Kidney showed hydronephrosis,normal left kidney
- Suprarenal areas are free
- Pelvis is occupied by huge multiloculated mass no solid component was noted.11‒15
Routine tests 18.09.08
- CBC & Blood Chemistry were Reported Normal values…
- Urine-analysis no specific finding,culture –ve
- Subsequent serials were WNL.
Lab. work up: 18.09.08
- LH 12.490mIU/ml
- FSH 1.120mIU/ml
- Prolactin 12.250ng/ml
- ééTestosterone 2.030ng/ml
- (N 0.060-0.820)
- DHEA-S 2.560µmol/L (1.770-9.990)
- 17-(OH) PG 7.80nmol/L
- (Luteal phase (N 0.6-8.8), Follicular (N 0.9-3.0), after ACTCH (less9))
- 18.09…
- Serum Cortisol Morning 9.270µg/dl (2.3-11.9)
- Free Androgen Index 47.60nmol/L (up to 11)
- SHBP 15 nmol/L
- ASD…
- TFT were WNL
Latent result…! 18.09
- ASD 7.250nmol/L (N 1.0-11.50)
- 17-(OH) PG 7.80nmol/L
(Luteal phase (0.6-8.8), Follicular (0.9-3.0), after ACTCH (less9))
- Testosterone 2.030ng/ml ( 0.060-0.820)
- DHEA-S 2.560µmol/L ( 1.770-9.990)
MRI of the pelvis
- A huge cystic mass measuring about 18x11cm is seen arising from the left ovary and displacing the uterus to right side.16
- Multiple thin septa are noted within the mass, no solid component seen, normal Right ovary seen, no adrenal mass, the right kidney shows hydronephrosis and hydroureter probably caused by back pressure from the ovarian mass, normal left kidney and other organ.17
Conclusion
The findings are suggestive of ovarian cystadenoma…
Gynecologic referral
We discussed the case with gynecologist…eventually; she decided to remove the cyst by exploring the pelvis, and looking for any source of Androgen Excess (virilism) that could be detected…!
Surgical intervention
Oct.28.08
- Gynecologist will take this action.
- Successful removal of the cyst, biopsy was taken from the Rt. Ovary (looks polycystic!).
- Uneventful recovery.
Hormonal Profile 30.10 Post-Op
- LH 6.670mIU/ml
- FSH 1.810mIU/ml
- Prolactin 12.430ng/ml
Testosterone 1.340ng/ml (0.060-0.820)éè
- DHEA-S 2.300µmol/L (1.770-9.990)
- Cortisol 12.300µg/dl
Latent…30.10
- 17 (OH) PG 4.70nmol/L (0.1-8.8)
- ASD (not received, sample problem!)
- SHBP 12.00nmol/L
- Free Androgen Index 33.80nmol/L (up to 11)
Hormonal profile 01.11
- LH 18.520mIU/ml
- FSH 4.800mIU/ml
- Prolactin 13.130ng/ml
- êTestosterone 0.287ng/ml (0.060-0.820)
- DHEA-S 1.490µmol/L
- Cortisol 11.970µg/dl ( 6.2-19.4 M)
Latent H.P 01.11
- 17 (OH) PG
- ASD sample …
- SHBP
- Free Androgen Index
Hormonal profile 20.11
- LH 9.510 mIU/ml
- FSH 3.510 mIU/ml
- Prolactin 7.770 ng/ml
- êêTestosterone 0.150 ng/ml (0.060-0.820)
- DHEA-S 1.740 µmol/L (1.770-9.990)
Hormonal Profile 20.11
- 17 (OH) PG
- ASD 5.50nmol/L (N 1.0-11.5); received on 19.01.09!
- SHBP
- Free Androgen Index
Histopathology Report
- Thin-walled multiloculated cyst 20x14x8cm, wt.1.5kg to its top attached a fallopian tube 7x0.6cm…
- Diagnosis:
- Serous Papillary Cystadenoma
- Normal Fallopian Tube
- Biopsy Report; Right Ovary
- A piece of greyish white tissue 1.5x0.4x0.9cm…
- Diagnosis
- Polycystic Ovary
- No evidence of malignancy…
Overview & Discussion
Large ovarian cysts are benign in majority of the cases and histopathologically these cysts are either serous or mucinous. Serous tumors secrete serous fluids and are originated by invagination of the surface epithelium of ovary. Serous tumors are commonly benign (70%); 5–10% have borderline malignant potential, and 20–25% are malignant. Only 10% cases of all serous tumors are bilateral. Serous cystadenomas are multilocular. Giant ovarian serous cyst adenoma is a rare finding. In the literature, a few cases of giant ovarian cysts have been mentioned and mostly in elderly postmenopausal women. In our context this big benign cystadenoma secretes androgen which is a rare characteristic of this epithelial tumor. Our presented case was a 16year old girl with features of virilizm and had no clinical complaint or signs of pelvic mass, except from nonspecific symptoms of anorexia and abdominal discomfort. The patient had been subjected to laparotomy by gynecologist and left salpingo-oophorectomy with cystectomy were done and all specimen with biopsy from right ovary (which showed polycystic) had been sent to histopathological examination which had confirmed the benign cystadenoma. The androgen excess had dropped remarkably post-operatively and overtime, as well as there were significant improvement in the virilizing features with subsequent follow-up visits, the patient had resumed her cycles and vocal and facial features had improves dramatically. Virilization is associated with a more severe degree of hyperandrogenism and should raise suspicion of an ovarian or adrenal neoplasm or other hormone-related condition such as polycystic ovary syndrome, adrenogenital syndrome (e.g. 21-Hyroxylae deficiency) and Cushing’s syndrome. Differential diagnoses of causes of hyperandrogenism will be listed down.18‒22
Androgen excess
- Androgen excess is the most common endocrine disorder in women of reproductive age ;
- Characterized by an excessive androgen production by the adrenal glands and/or the ovary, androgen excess may result from increased local tissue sensitivity to circulating androgens.
- Androgen excess affects different tissues and organ systems, causing clinical conditions ranging from acne to hirsutism to frank virilization…….Sources of androgens in women
- Androgen sources in women are the endocrine glands (adrenal glands and ovaries) and peripheral tissues such as fat and skin…
- Liver and gut play a minor role in androgen production, particularly in the peripheral conversion of testosterone to the most active form dihydrotestosterone (DHT).
Androgens: The endocrine glands secrete 5 androgens through a similar pathway:
- Dehydroepiandrosterone sulphate (DHEAS)
- Dehydroepiandrosterone (DHEA)
- Androstenedione (ASD)
- Testosterone
- Androstenediol (has both androgenic and estrogenic activity).
- Testosterone is the only androgen with direct androgenic activity, while DHEAS, DHEA, & Androstenedione (ASD) are all precursors of testosterone…
- The Ovaries (Figures 1&2)
- The ovaries, under the control of luteinizing hormone (LH), produce 50% of the total testosterone that rises to 75% at midcycle.
- The ovaries also secrete 50% of the total androstenedione and small amounts (20%) of DHEA.
- Testosterone is used as a marker of ovarian androgen secretion; however, the adrenals, via peripheral conversion of androstenedione to testosterone, also contribute to total testosterone.
- The Adrenals
- The adrenal glands produce all the DHEAS and 80% of the DHEA.
- The adrenals also secrete 50% of androstenedione and 25% of the circulating levels of testosterone.
- DHEAS and 11-androstenedione are not secreted by the ovaries and, therefore, are used as markers of adrenal androgen secretion.
- Their secretion depends on (ACTH); prolactin and estrogen can affect adrenal androgen production…
- Peripheral Tissues
- Skin, fat, liver, and urogenital systems are important peripheral sites of androgen production.
- Androstenedione, and to some degree DHEA, are converted to testosterone in the skin…
- Androgens…
- DHT is an intracrine hormone that is produced, acts, and is metabolized within the target tissues.
- DHT is produced by action of the 5α-Reductase enzymes on testosterone, androstenedione, and DHEA…
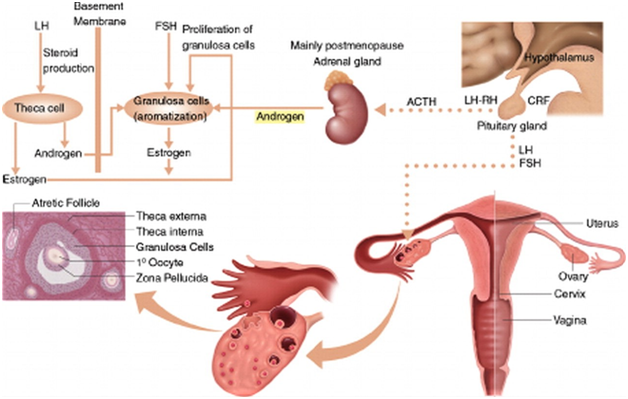
Figure 1 Synthesis of sex steroids, in women, androgens and their precursors are produced by both the adrenal glands and the ovaries in response to their respective ACTH and luteinizing hormone (LH). In the ovaries, androgens are produced as precursors in the synthesis of estrogen and estradiol. The theca cells of preantral (secondary) ovarian follicles produce and rostenedione and testosterone in response to luteinizing hormone. (Courtesy of; Functioning Ovarian Tumors: Direct and Indirect Findings at MR Imaging: Yumiko O. Tanaka et al.)
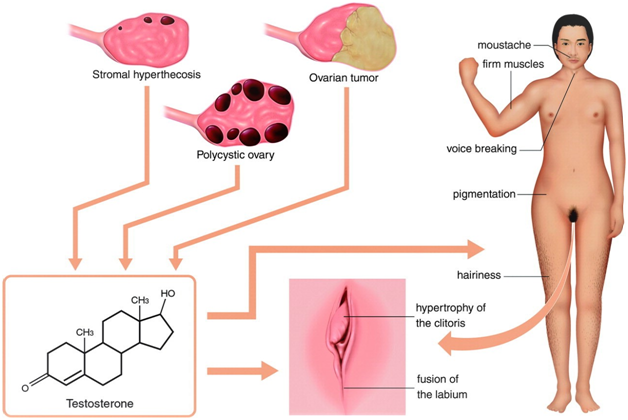
Figure 2 Hyperandrogenism in women. An excess of androgens causes hirsutism and, in severe cases, virilization. Hirsutism involves the presence of hair that does not normally appear in women, such as a mustache. Virilization includes male-pattern baldness, coarsening of the voice, a decrease in breast size, an increase in muscle mass, loss of female body contour, and enlargement of the clitoris. (The same previous source).
Ovarian pathology
Ovarian androgen-secreting tumors
- Sertoli-Leydig cell tumors
- Leydig cell tumors
- Lipoid or lipid cell tumors
- Granulosa-theca cell tumors
- Hilus cell tumors
- Gynandroblastoma
- Steroid cell tumors
- Teratoma
- Gonadoblastoma
Excessive androgen
- Polycystic ovarian syndrome (PCOS) is the most common hyperandrogenic disorder, affecting 5-10% of all women.
- PCOS involves irregular ovulation in combination with excess androgens and with or /wo polycystic ovaries on imaging.
- Hyperthecosis, can have testosterone levels in the tumor range!
- Tumors of the ovary secrete high levels of testosterone.
The testosterone levels exceed 2.0ng/mL (200ng/dL, 8.92nmol/L) or 2.5times the upper limit of the reference range; Sertoli-Leydig cell tumors, hilus cell tumors, and lipoid cell (adrenal rest) tumors are the most common.
Adrenal pathology
- Tumors of the adrenal glands (adenomas, carcinomas), which secrete elevated levels of androgens, are known but rare.
- They are suspected when DHEAS exceeds
- 7μg/mL (18μmol/L).
- Classical & non-classical (late-onset) CAH
- Cushing syndrome: Patients secrete elevated androgens, but diagnosing this disease by hyperandrogenic manifestations without the other signs and symptoms of Cushing syndrome would be unusual.
The patient‘s testosterone; serials
- 2.68è (Post-Op) 2.030 ---Post-Op--è 1.340 è0.287è0.150
Female ref. (0.060-0.820ng/ml)
Corresponding drop in Free Androgen Index & other androgenic parameters.
Endocrine diagnosis
PCOS with Ovarian Cyst (Androgen- secreting…)
Management:
- Induction of the Cycle
- Oral Contraceptive Combination ( Estrogen-progestin)
- Spironolactone
- Metformin
Patient had been feeling well and her voice was noticeably improved.
Acknowledgments
Conflicts of interest
The authors declare that there is no conflict of interest.
Funding
References
- Mishra S, Yadav M, Walawakar SJ. Giant Ovarian Mucinous Cystadenoma Complicating Term Pregnancy. JNMA J Nepal Med Assoc. 2018;56(210):629‒632.
- Bell DA, Scully RE. Atypical and borderline endometrioid adenofibromas of the ovary. A report of 27 cases. Am J Surg Pathol. 1985;9(3):205‒214.
- Seidman JD, Krishnan J. Ovarian Epithelial Inclusions With Mucinous Differentiation: A Clinicopathologic Study of 42 Cases. Int J Gynecol Pathol. 2017;36(4):372‒376.
- Jung EJ, Eom HM, Byun JM, et al. Different features of the histopathological subtypes of ovarian tumors in pre‒ and postmenopausal women. Menopause. 2017;24(9):1028‒1032.
- Hunter SM, Anglesio MS, Sharma R, et al. Longacre TA, Huntsman DG, Gorringe KL, Campbell IG. Copy number aberrations in benign serous ovarian tumors: a case for reclassification? Clin. Cancer Res. 2011;17(23):7273‒7282.
- Seidman JD, Khedmati F. Exploring the histogenesis of ovarian mucinous and transitional cell (Brenner) neoplasms and their relationship with Walthard cell nests. Arch Pathol Lab Med. 2008;132(11):1753‒1760.
- Cuatrecasas M, Villanueva A, Matias‒Guiu X, et al. K‒ras mutations in mucinous ovarian tumors: a clinicopathologic and molecular study of 95 cases. Cancer. 1997;79(8):1581‒1586.
- Poli Neto OB, Candido Dos Reis FJ, Zambelli Ramalho LN, et al. p63 expression in epithelial ovarian tumors. Int J Gynecol Cancer. 2006;16(1):152‒155.
- Seidman JD, Mehrotra A. Benign ovarian serous tumors: a re‒evaluation and proposed reclassification of serous "cystadenomas" and "cystadenofibromas". Gynecol Oncol. 2005;96(2):395‒401.
- Massicot R, Rousseau V, Darwish AA, et al. Serous and seromucinous infantile ovarian cystadenomas‒‒a study of 42 cases. Eur J Obstet Gynecol Reprod Biol. 2009;142(1):64‒67.
- Fatema N, Mubarak Al Badi M. A Postmenopausal Woman with Giant Ovarian Serous Cyst Adenoma: A Case Report with Brief Literature Review. Case Rep Obstet Gynecol. 2018;2018:5478328.
- Jeong YY, Outwater EK, Kang HK. Imaging evaluation of ovarian masses. Radiographics. 2000;20(5):1445‒1470.
- Buy JN, Ghossain MA, Sciot C, et al. Epithelial tumors of the ovary: CT findings and correlation with US. Radiology. 1991;178(3):811‒818.
- Gonzalez DO, Minneci PC, Deans KJ. Management of benign ovarian lesions in girls: a trend toward fewer oophorectomies. Curr Opin Obstet Gynecol. 2017;29(5):289‒294.
- Cohen I, Nabriski D, Fishman A. Noninvasive test for the diagnosis of ovarian hormone‒secreting‒neoplasm in postmenopausal women. Gynecol Oncol Rep. 2016;15:12–15.
- Burnik Papler T, Frković Grazio S, et al. Sertoli‒Leydig cell tumor with retiform areas and overgrowth of rhabdomyosarcomatous elements: case report and literature review. J Ovarian Res. 2016;9(1):46.
- Stavrakis T, Kalogiannidis I, Petousis S, et al. Fertility‒sparing management and obstetric outcomes in a 20‒year‒old patient with a Sertoli‒Leydig cell tumor of the ovary: A case report and review of the literature. Oncol Lett. 2016;12(2):1079–1082.
- Ting Y, Yang LI, Juan Z, et al. Ovarian thecoma with massive pleural effusion in postmenopausal women: A case report. Mol Clin Oncol. 2015;4(6):1003–1005.
- Park JW, Bae JW. Postmenopausal Meigs’ Syndrome in elevated CA‒125: a case report. J Menopausal Med. 2015;21(1):56–59.
- Ramkumar S, Jyotsna VP, Mallick S, et al. Bilateral thecoma presenting as premenopausal hirsutism: Laproscopic removal. Indian J Endocrinol Metab. 2013;17(Suppl 3):S662–664.
- Atram M, Anshu, Sharma S, Gangane N. Sclerosing stromal tumor of the ovary. Obstet Gynecol Sci. 2014;57(5):405–408.
- Tian T, Zhu Q, Chen W, et al. CT findings of sclerosing stromal tumor of the ovary: A report of two cases and review of the literature. Oncol Lett. 2016;11(6):3817–3820.
- Horta M, Cunha TM. Sex cord‒stromal tumors of the ovary: a comprehensive review and update for radiologists. Diagn Interv Radiol. 2015;21(4):277–286.
- Sood N, Desai K, Chindris A‒M, et al. Symptomatic ovarian steroid cell tumor not otherwise specified in a post‒menopausal woman. Rare Tumors. 2016;8(2):6200.
- Bettegowda A, Rangaiah N, Prasad N, at al. Virilizing ovarian steroid cell tumor: a rare case. J Clin Diagn Res. 2015;9(9):QD05–QD06.
- Iqbal A, Novodvorsky P, Lubina‒Solomon A, et al. Juvenile granulosa cell tumour of the ovary presenting with hyperprolactinaemic amenorrhoea and galactorrhoea. Endocrinol Diabetes Metab Case Rep. 2016;2016:160006.
- Vasu PP, Leelamma JP, Mohammed BA, et al. Primary granulosa cell tumor of retroperitoneal origin: A rare presentation with emphasis on cytomorphology. J Cytol. 2016;33(1):52–54.
- Alexiadis M, Chu S, Leung D, et al. Transcriptomic analysis of stage 1 versus advanced adult granulosa cell tumors. Oncotarget. 2016;7(12):14207–14219.
- Hattori Y, Yamada S, Yamamoto M, et al. Ovar an mucinous adenocarcinoma with functioning stroma in postmenopausal women: aromatase and SF‒1 expressions. J Ovarian Res. 2015;8:73.

©2020 AlJadir. This is an open access article distributed under the terms of the,
which
permits unrestricted use, distribution, and build upon your work non-commercially.




