eISSN: 2473-0815


Background: Total 25-hydroxyvitamin D 25(OH)D levels are influenced by vitamin D binding globulin (VDBG) produced by hepatocytes.
Aim: To determine associations of VDBG with vitamin D measures and associations of total and calculated free 25(OH)D with albumin corrected calcium and intact parathyroid hormone (iPTH) levels in the setting of cirrhosis.
Methods: Correlation coefficients were calculated between log transformed VDBG and vitamin D measures. Linear regression models were constructed to examine the associations of total and free 25(OH)D levels with albumin corrected serum calcium and iPTH levels in 40 patients with cirrhosis.
Results: Median age was 60.1 years and cirrhosis was attributed to hepatitis C in 47.5%, alcohol use in 27.5%, and other in 25%. Median total 25(OH)D, calculated free 25(OH)D, and VDBG levels were 18.4 (13.1, 32.7) ng/mL, 10.2 (7.4, 13.4) pg/mL, and 143.8 (106.7, 205.2) µg/mL, respectively. VDBG showed strong correlations with 25(OH)D (r=0.64; P < 0.001) but weak correlations with free 25(OH)D (r=0.15; P =0.4). Albumin corrected serum calcium showed moderate correlations with total (r=0.24; P =0.1) and free 25(OH)D (r=0.34; P 0.01) but not with VDBG levels (r=-0.05; P =0.8). In contrast, iPTH levels showed a weak correlation with 25(OH)D (r=0.14; P =0.4) and free 25(OH)D (r=0.02; P =0.9). After adjustment for covariates, free 25(OH)D was significantly associated with albumin corrected serum calcium levels (β= 0.005; 95% CI 0.0004, 0.009) but not with iPTH (β= -0.006; 95% CI -0.04, 0.03).
Conclusion: In adults with cirrhosis, total but not free 25(OH)D is associated with VDBG levels.
Keywords: vitamin D binding globulin, vitamin D, free vitamin D, bioavailable D, cirrhosis, liver disease
Cirrhosis is often accompanied by low 25-hydroxyvitamin [25(OH)D] levels (<20 ng/ml),1–4 which indicates insufficient or deficient vitamin D status.5 Inadequate vitamin D status among adults with liver disease may be attributed to multiple factors including lack of liver hydroxylation of calciferol to yield 25(OH)D, low sunlight exposure, malabsorption or other factors. Vitamin D undergoes its first hydroxylation step in the liver, forming 25(OH)D, which is then further hydroxylated to its active form, 1,25 dihydroxyvitamin D [1,25(OH)2D], primarily in the kidneys.6 This active form of vitamin D impacts calcium homeostasis by increasing bone mineralization and intestinal calcium absorption.9,10 To prevent accumulation of toxic levels of vitamin D, the gene CYP24A1 encodes an enzyme that catalyzes the 24-hydroxylation of 25(OH)D and its active form 1,25-dihydroxyvitamin D [1,25(OH)2D), converting them to inactive forms.6
Vitamin D status holds clinical importance in the setting of liver disease because low 25(OH)D levels may negatively impact immune function and bone formation and possibly influence liver disease progression.7–12 However, assessment of vitamin D status in the setting of cirrhosis is complicated by the fact that approximately 90% of 25(OH)D levels circulate bound to VDBG, a protein produced by hepatocytes.13 Low levels of 25(OH)D, the major circulating form of vitamin D, may reflect impaired production of VDBG by hepatocytes.(4, 14) Free and bioavailable 25(OH)D have been proposed as alternative measures for assessment of vitamin D status because free 25(OH)D circulates unbound while bioavailable circulates free or only weakly bound to albumin.15,16 Thus, both free and bioavailable 25(OH)D are not dependent on VDBG production.
Despite low vitamin D levels in patients with liver disease, we and others have previously shown that 25(OH)D levels do not correlate with albumin corrected serum calcium levels in patients with cirrhosis.4,17,18 Moreover, hypocalcemia and high intact parathyroid hormone (iPTH) levels are uncommon among adults with cirrhosis and low 25(OH)D levels which suggests that compensatory mechanisms exist.3,4,18,19 Previous studies have reported higher levels of free 25(OH)D levels in adults with cirrhosis compared to levels in healthy adults and pregnant women.19,20 Another alternative measure is the ratio of 24,25(OH)2D3-to-25(OH)D. In the setting of low vitamin D status, inactivation of vitamin D will be low. Higher levels of the ratio 24,25(OH)2D3-to-25(OH)D indicate upregulation of the gene CYP24A1 to prevent vitamin D intoxication. Few studies have examined this ratio, especially in the setting of cirrhosis. In this pilot study, we measured VDBG and total 25(OH)D, 1,25(OH)2D and the ratio of 24,25(OH)2D3-to-25(OH)D and calculated free and bioavailable 25(OH)D in 40 adults with cirrhosis to investigate the associations of VDBG with total, free and bioavailable 25(OH)D and the associations of vitamin D measures with albumin-corrected serum calcium and iPTH levels in patients with cirrhosis.
Study population
This cross-sectional study included patients with cirrhosis receiving care at Loyola University Medical Center, a large, urban academic medical institution. The 40 patients were selected from a cohort study of 456 patients with cirrhosis designed to examine the determinants of liver disease progression. Patients were recruited during a routine clinic visit from August 29, 2013 to January 28, 2015 when clinical; biochemical and radiological tests were performed. Diagnosis of cirrhosis was based on clinical and radiological data or examination of liver histology. We selected 40 participants with a range of modeling end-stage liver disease-sodium (MELD-Na) scores and liver disease due to hepatitis C and/or alcohol use or non-alcoholic steatohepatitis (NASH), the three most common causes of cirrhosis, and with complete information on serum calcium, albumin, and creatinine measurements. The study was approved by the Loyola University Chicago Institutional Review Board and all patients provided written informed consent.
Vitamin D binding globulin
Liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) methodology was used to measure VDBG. The lower limit of quantification was 71μg/ml and the assay was linear from 62 to 434 μg/ml with a CV of 7.3-9.0%. Mean concentration (standard deviation) in healthy adults using this assay has been reported as 262 (25) μg/ml.21 Unlike immunoassays, this method does not use antibodies that recognize regions of the protein that may be affected by the genotype of GC which encodes VDBG, a highly polymorphic protein. Thus, the GC genotype does not influence this assay.21 In addition; previous validation has demonstrated that bilirubin (conjugated and unconjugated) does not have an effect on the performance of the assay.21
Vitamin D and biochemical measures
Non-fasting blood specimens were collected during a clinic visit and were sent immediately to the Loyola laboratory for measurement of routine clinical measurements. Levels of 25(OH)D, 1,25(OH)2D and 24,25(OH)2D3 were measured in serum using immunoaffinity extraction and liquid chromatography/tandem mass spectrometry (LC-MS/MS). Calibration of 25(OH)D was verified using National Institute of Standards Technology (NIST) SRM 972a. Levels of free 25(OH)D and bioavailable 25(OH)D were calculated based on previously reported affinity binding constants between 25(OH)D, VDBG, and albumin.22 Levels of iPTH were measured in serum using an automated sandwich immunoassay (Beckman Coulter DxI). Levels of iPTH, 25(OH)D, 1,25(OH)2D, 24,25(OH)2D3, and VDBG were measured at the University of Washington Department of Laboratory Medicine, Seattle, Washington Serum albumin, creatinine and total bilirubin levels were measured using a colorimetric method and calcium was measured using indirect ion selective electrodes. Blood reference range values for serum calcium and albumin were 8.5-10.5 mg/dl and 3.6-5.0 g/dl, respectively. The following equation was used to calculate corrected calcium levels: corrected Ca (mg/dl) = measured total calcium in mg/dl + [0.8 x (4–albumin in g/dl])]. Serum sodium was measured using an ion specific electrode. Serum albumin, creatinine, total bilirubin, international normalized ratio (INR), and sodium were measured at the Loyola University Medical Center clinical laboratory.
Liver disease severity
Liver disease severity was assessed with serum albumin levels23 and the MELD-Na score.24 The original MELD score predicted prognosis in patients with cirrhosis based on kidney function and dialysis requirements, serum bilirubin and the international normalized ratio (INR). In recognition of the independent association between serum sodium and transplant waitlist mortality for patients with cirrhosis,24–28 the Organ Procurement and Transplantation Network began incorporating the sodium into the MELD score for prioritizing transplants based on liver disease severity in 2016.29 Higher MELD-Na scores indicate increased severity of liver disease.
Disease etiology, demographics
Information on demographics was obtained using standardized questionnaires, while the primary etiology of liver disease was obtained from the electronic medical record. At the clinic visit, weight was measured without shoes to the nearest 0.1 kg using a standard balance and height was measured using a stadiometer without shoes. Body mass index (BMI) was calculated as weight in kg divided by the height in meters squared. Information on vitamin D supplementation, including ergocalciferol or cholecalciferol, and calcium supplementation was obtained from the medication list in the electronic medical record at the time of enrollment.
We used STATA/IC 13.1 (StataCorp LP, College Station, TX, USA) to perform all statistical analyses. Median values (interquartile range) are presented for continuous variables, and frequencies were reported for categorical variables. Scatterplots of serum levels of VDBG vs. MELD-Na scores and serum albumin and vs. vitamin D measures were examined. Pearson correlation coefficients were calculated to quantify the correlation between log transformed values of VDBG, vitamin D measures and serum albumin corrected calcium and iPTH levels. Linear regression models were then used to examine the factors that account for the variance in log transformed 25(OH)D levels in this sample of adults with liver disease. Models examined demographic factors age, sex, race and body mass index (BMI), serum creatinine levels, and use of vitamin D supplements. These covariates were selected because they may influence vitamin D levels.1,30 Linear regression models were also constructed to examine the association of vitamin D measures with log transformed albumin corrected calcium and iPTH levels. The models adjusted for variables selected due to their potential influence on calcium and iPTH levels including age, sex, race, BMI, serum creatinine level, and use of vitamin D or calcium supplements. Regression analyses were repeated after excluding individuals using calcium or vitamin D supplements (n=10).
The median age of the 40 adults with cirrhosis was 60.1 (55.3-64.7) years. The majority (62.5%) were white and over half (52.5%) were obese see Table 1. Cirrhosis was attributed to hepatitis C in 47.5%, alcohol use in 27.5%, both hepatitis C and alcohol use in 17.5% and NASH in 7.5%. Vitamin D supplementation use was reported by 30% of patients while only 10.8% were using calcium supplements. The median MELD-Na score was 13.0 (Interquartile Range [IQR] 9.5-16.5) with a range of 6-30 and median VDBG levels were 142.8 (IQR 106.7-205.2) μg/ml. The median 25(OH)D and 1,25(OH) 2D levels were 18.4 (IQR 13.1-32.7) ng/ml and 36.4 (IQR 26.6-49.4) pg/ml, respectively while median free and bioavailable 25(OH)D levels were 10.2 (IQR 7.4, 13.4) pg/mL, and 3.0 (IQR 2.1, 4.2) µg/mL, respectively. The normal reference range for iPTH was 10-65 pg/ml and the median iPTH level in this group was 25.3 (IQR 18.8-31.3) pg/ml.
Age (years) |
60.1 (55.3-64.7) |
% Male |
55 (n=22) |
Race |
|
% White |
62.5 (n=25) |
% Black |
37.5 (n=15) |
BMI (kg/m2) |
30.5 (24.9, 33.8) |
Cirrhosis etiology |
|
% NASH |
7.5 (n=3) |
% ETOH |
27.5 (n=11) |
% HCV |
47.5 (n=19) |
% ETOH and HCV |
17.5 (n=7) |
MELD-Na |
13.0 (9.5-16.5) |
Intact parathyroid hormone (pg/mL) |
25.3 (18.8-31.3) |
*Calcium (mg/dL) |
9.54 (9.23-9.84) |
Albumin (g/dL) |
3.2 (2.8-3.8) |
Serum creatinine (mg/dl) |
0.86 (0.72-1.00) |
25-hydroxyvitamin D (ng/ml) |
18.4 (13.1-32.7) |
1,25(OH)2D (pg/mL) |
36.4 (26.6-49.4) |
24,25(OH)2D3 (ng/ml) |
0.71 (0.31-1.29) |
Ratio 24,25(OH)2D3-to-total 25(OH)D |
0.04 (0.02-0.06) |
Vitamin D binding globulin (µg/mL) |
143.8 (106.7-205.2) |
Free 25(OH)D (pg/mL) |
10.2 (7.4, 13.4) |
Bioavailable 25(OH)D (µg/mL) |
3.0 (2.1, 4.2) |
% Using vitamin D supplements |
30.0 (n=12) |
% Using calcium supplements |
10.8 (n=4) |
Table 1 Baseline characteristics of study participants (n=40)
Data presented as median (interquartile range) or %. Free vitamin D and bioavailable D are calculated based on affinity constants for 25(OH)D and VDBG and albumin reported by Bikle et al.20
Abbreviations: BMI, body mass index; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 24,25(OH)2D3, 24,25 dihydroxyvitamin D3; MELD-Na, modeling end-stage liver disease score; NASH, non-alcohol steatohepatitis; ETOH, alcoholic liver disease; HCV, hepatitis C virus, *Calcium is adjusted for serum albumin levels.
Figure 1 shows the scatter plots of VDBG by MELD-Na scores and by serum albumin levels. The upper figure with MELD-Na score on the horizontal axis shows an outlier with a VDBG of 220 μg/ml and a MELD-Na score of 28. This patient had a serum total bilirubin level of 0.8 mg/dl and an INR of 1.21 and serum albumin level of 3.1 mg/dl. However, the serum creatinine was 8.58 mg/dl which raised the MELD-Na score. The next highest creatinine value in the study population was 2.25 and the majority of patients had serum creatinine values < 1.0 mg/dl. VDBG was strongly and significantly correlated with both MELD-Na (r=-0.51; P < 0.001) and serum albumin levels (r=0.54; P <0.001). After excluding the patient with a serum creatinine of 8.58 mg/dl, the correlation coefficients between VDBG and MELD-Na scores (r= -0.62; P < 0.001) and serum albumin (r= 0.55; P < 0.001) were somewhat stronger. Figure 2 shows the scatterplots of the vitamin D measures by VDBG levels. Fairly linear distributions were noted between 25(OH)D and 1,25(OH)2D and VDBG levels. Table 2 shows the correlations between the log transformed VDBG and vitamin D measures, albumin, calcium and iPTH levels. Strong and significant correlations were noted between VDBG and 25(OH)D (r= 0.64; P < 0.001) and 1,25(OH)2D (r = 0.53; P < 0.001) while weaker correlations were noted between VDBG and free (r=0.15; P = 0.4) and bioavailable 25(OH)D (r=0.34; P =0.06). Albumin corrected serum calcium showed moderate correlations with total (r=0.24; P =0.1), free (r=0.34; P 0.01) and bioavailable 25(OH)D (r=0.29; P =0.07) while iPTH levels showed moderate correlations only with VDBG (r= -0.22; P = 0.1) and the ratio of 24,25(OH)2D3-to-25(OH)D (r= -0.29; P=0.07). Results did not change substantially after excluding individuals receiving vitamin D or calcium supplements.
Total sample (n=40) |
||||||||
|
VDBG |
25(OH)D |
1,25(OH)2D |
Free 25(OH)D |
Bioavail 25(OH)D |
24,25(OH)2D3-to-25(OH)D |
Calcium |
iPTH |
VDBG |
1.0 |
|
|
|
|
|
|
|
25(OH)D |
0.64* |
1.0 |
|
|
|
|
|
|
1,25(OH)2D |
0.53* |
0.40+ |
1.0 |
|
|
|
|
|
Free 25(OH)D |
0.15 |
0.86* |
0.16 |
1.0 |
|
|
|
|
Bioavail 25(OH)D |
0.34 |
0.91* |
0.26 |
0.93* |
1.0 |
|
|
|
24,25(OH)2D-to-25(OH)D3 |
0.33 |
0.30 |
0.25 |
0.15 |
0.26 |
1.0 |
|
|
Calcium |
-0.05 |
0.24 |
-0.10 |
0.34+ |
0.29 |
-0.04 |
1.0 |
|
iPTH |
-0.22 |
0.14 |
0.07 |
0.02 |
-0.05 |
-0.29 |
-0.22 |
1.0 |
|
||||||||
Non-users of vitamin D or calcium supplements (n=30) |
||||||||
|
||||||||
|
VDBG |
25(OH)D |
1,25(OH)2D |
Free 25(OH)D |
Bioavail 25(0H)D |
24,25(OH)2D3-to-25(OH)D |
Calcium |
iPTH |
VDBG |
1.0 |
|
|
|
|
|
|
|
25(OH)D |
0.65* |
1.0 |
|
|
|
|
|
|
1,25(OH)2D |
0.65* |
0.36+ |
1.0 |
|
|
|
|
|
Free 25(OH)D |
0.16 |
0.86* |
0.23 |
1.0 |
|
|
|
|
Bioavail 25(OH)D |
0.35+ |
0.91* |
0.34+ |
0.93* |
1.0 |
|
|
|
24,25(OH)2D3-to-25(OH)D |
0.42+ |
0.34 |
0.21 |
0.14 |
0.29 |
1.0 |
|
|
†Calcium |
-0.03 |
0.33+ |
-0.20 |
0.39+ |
0.38+ |
-0.05 |
1.0 |
|
iPTH |
0.22 |
-0.03 |
0.29 |
-0.16 |
-0.23 |
-0.28 |
-0.17 |
1.0 |
Table 2 Pearson correlation matrix of log transformed measures of vitamin D, parathyroid hormone, and calcium levels in adults with liver disease (n=40) and after excluding users of vitamin D or calcium supplements (n=30)
†Calcium, measured total calcium in mg/dL= [0.8 x (4 – albumin in g/dL)].
Abbreviations: VDBG, vitamin D binding globulin; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; Free D, 25(OH)D not bound to or albumin; Bioavail 25(OH)D, bioavailable 25(OH)D that is free or weakly bound to albumin; VDBG, vitamin D binding protein; 24,25(OH)2D, 24,25-dihydroxyvitamin D; iPTH, intact parathyroid hormone level; *P < 0.001; +P < 0.05
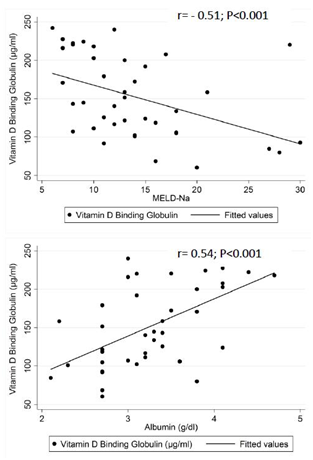
Figure 1 Scatterplots of vitamin D binding globulin by modeling end-stage liver diseae sodium (MELD-Na) scores (top) and by serum albumin levels (bottom) in 40 adults with cirrhosis.

Figure 2 Scatterplots of 25-hydroxyvitamin D [25(OH)D] (upper left), 1,25-dihydroxyvitamin D [1,25(OH)2D (upper right), free 25-hydroxyvitamin D (bottom left) and bioavailable 25-hydroxyvitamin D (bottom right) vs. vitamin D binding globulin levels in 40 adults with cirrhosis.
The results of the linear regression analyses with log transformed 25(OH)D as the dependent variable are shown in Table 3. VDBG levels alone accounted for 41% of the variance in 25(OH)D levels. The addition of demographic variables and use of vitamin D supplements did not substantially increase the R2 value. The only variable significantly associated with log transformed 25(OH)D level was VDBG. Findings did not change after excluding the 10 individuals using calcium or vitamin D supplements which included the one patient with a serum creatinine of 8.58 mg/dl.
|
Model 1 |
|
|
Model 2 |
|
|
Total sample (n=40) |
||||||
|
|
R2 |
P Value |
|
R2 |
P Value |
Vitamin D binding globulin |
0.01 (0.005, 0.01) |
0.41 |
<0.001 |
0.01 (0.005, 0.01) |
0.44 |
<0.001 |
Age (per year increase) |
0.01 (-0.01, 0.04) |
0.03 |
0.3 |
-0.01 (-0.03, 0.02) |
|
0.7 |
Male vs. female |
-0.29 (-0.70, 0.11) |
0.05 |
0.2 |
-0.22 (-0.56, 0.12 |
|
0.2 |
White race vs. non-white race |
-0.33 (-0.74, 0.09) |
0.06 |
0.1 |
0.03 (-0.40, 0.46) |
|
0.9 |
BMI (per kg/m2 increase) |
0.004 (-0.02, 0.03) |
0.002 |
0.8 |
0.01 (-0.02, 0.03) |
|
0.5 |
Vitamin D supplementation vs. no supplementation |
0.10 (-0.35, 0.55) |
0.01 |
0.7 |
-0.08 (-0.05, 0.36) |
|
0.7 |
Non-users of Vitamin D or Calcium supplements (n=30) |
||||||
|
||||||
Vitamin D binding globulin |
0.01 (0.006, 0.01) |
0.51 |
<0.001 |
0.01 (0.004, 0.01) |
0.58 |
0.001 |
Age (per year increase) |
0.01 (-0.02, 0.05) |
0.04 |
0.3 |
-0.01 (-0.03, 0.02) |
|
0.6 |
Male vs. female |
-0.55 (-1.11, 0.02 |
0.13 |
0.06 |
-0.40 (-0.86, 0.06) |
|
0.09 |
White race vs. non-white race |
-0.53 (-1.17, 0.10) |
0.10 |
0.10 |
-0.17 (-0.73, 0.39) |
|
0.6 |
BMI (per kg/m2 increase) |
0.003 (-0.05, 0.06) |
0.00 |
0.9 |
-0.002 (-0.04, 0.04) |
|
0.9 |
Table 3 Results of linear regression analyses with log transformed 25-hydroxyvitamin D levels as dependent variable
Model 1 is unadjusted; Model 2 is mutually adjusted for all variables in table.
Table 4 shows the associations of vitamin D measures with albumin corrected serum calcium levels. In both unadjusted and adjusted models, only free and bioavailable 25(OH)D were significantly associated with albumin corrected serum calcium levels. Results did not change after excluding individuals using calcium or vitamin D supplements. In contrast, no vitamin D measure was significantly associated with iPTH levels after adjusting for covariates (Table 5).
|
Model 1 |
|
|
Model 2 |
|
|
Total Sample (n=40) |
||||||
|
|
R2 |
P Value |
|
R2 |
P Value |
Free 25(OH)D |
0.004 (0.001, 0.01 |
0.12 |
0.03 |
0.005 (0.0004, 0.01) |
0.29 |
0.03 |
Bioavailable 25(OH)D |
0.01 (0.002, 0.03) |
0.13 |
0.03 |
0.01 (0.0004, 0.03) |
0.28 |
0.04 |
25(OH)D |
0.001 (-0.001, 0.002) |
0.04 |
0.2 |
0.001 (-0.001. 0.002) |
0.20 |
0.3 |
1,25(OH)2D |
-0.0002 (-0.001, 0.001) |
0.01 |
0.6 |
-0.00 (-0.0001, 0.001) |
0.20 |
0.8 |
24,25(OH)2D3-to-25(OH)D |
-0.21 (-1.05, 0.62) |
0.01 |
0.6 |
-0.28 (-1.33, 0.76) |
0.16 |
0.6 |
Non-users of Vitamin D or Calcium supplements (n=30) |
||||||
Free 25(OH)D |
0.01 (0.0004, 0.01) |
0.17 |
0.03 |
0.004 (-0.001, 0.01) |
0.23 |
0.08 |
Bioavailable 25(OH)D |
0.02 (0.004, 0.03) |
0.21 |
0.01 |
0.02 (0.001, 0.03) |
0.26 |
0.04 |
25(OH)D |
0.001 (-0.001, 0.003) |
0.05 |
0.3 |
0.0009 (-0.001, 0.003) |
0.14 |
0.4 |
1,25(OH)2D (per unit increase) |
-0.0004 (-0.002, 0.001) |
0.02 |
0.5 |
-0.0004 (-0.002, 0.001) |
0.13 |
0.6 |
24,25(OH)2D3-to-25(OH)D |
-0.28 (-1.47, 0.92) |
0.009 |
0.6 |
-0.36 (-1.58, 0.87) |
0.13 |
0.6 |
Table 4 Unadjusted (Model 1) and adjusted (Model 2) associations of vitamin D measures with log transformed serum calcium levels in adults with cirrhosis
Model 1 is unadjusted; Model 2 adds age, sex, race, body mass index (BMI), serum creatinine and use of vitamin D or calcium supplements (in total sample only);
|
Model 1 |
|
|
Model 2 |
|
|
Total Sample (n=40) |
|
|
|
|
|
|
|
|
R2 |
P Value |
|
R2 |
P Value |
Free 25(OH)D |
0.008 (-0.03, 0.04) |
0.06 |
0.7 |
-0.006 (-0.04, 0.03) |
0.39 |
0.7 |
Bioavailable 25(OH)D |
-0.007 (-0.12, 0.11) |
0.0003 |
0.9 |
-0.03 (-0.14, 0.08) |
0.39 |
0.6 |
25(OH)D |
0.01 (-0.002, 0.02) |
0.06 |
0.1 |
0.004 (-0.01, 0.02) |
0.39 |
0.5 |
1,25(OH)2D |
0.002 (-0.006, 0.01) |
0.6 |
0.01 |
0.001 (-0.01, 0.01) |
0.39 |
0.9 |
24,25(OH)2D3-to-25(OH)D |
-7.50 (-14.83, -0.17) |
0.10 |
0.05 |
-5.61 (-14.04, 2.82) |
0.42 |
0.2 |
Non-users of Vitamin D or Calcium supplements (n=30) |
||||||
Free 25(OH)D |
0.02 (-0.03, 0.06) |
0.03 |
0.4 |
-0.003 (-0.05, 0.04) |
0.38 |
0.9 |
Bioavailable 25(OH)D |
0.04 (-0.11, 0.18) |
0.01 |
0.6 |
-0.02 (-0.16, 0.13) |
0.39 |
0.8 |
25(OH)D |
0.01 (-0.001, 0.03) |
0.13 |
0.06 |
0.005 (-0.01, 0.02) |
0.39 |
0.6 |
1,25(OH)2D |
0.003 (-0.01, 0.01) |
0.01 |
0.6 |
-0.001 (-0.01, 0.01) |
0.38 |
0.8 |
24,25(OH)2D3-to-25(OH)D |
-6.23 (-17.16, 4.61) |
0.05 |
0.2 |
-4.92 (-14.55, 4.71) |
0.41 |
0.3 |
Table 5 Unadjusted (Model 1) and adjusted (Model 2) associations of vitamin D measures with log transformed intact parathyroid hormone levels in adults with cirrhosis
Model 1 is unadjusted; Model 2 adds age, sex, race, body mass index (BMI), serum creatinine and use of vitamin D and calcium supplements (in total sample only).
This pilot study shows that VDBG levels are quite low in adults with cirrhosis and levels correlate with liver disease severity as assessed by the MELD-Na score. Our findings are supported by a previous study demonstrating low VDBG levels in adults awaiting liver transplant.4 The main role of VDBG is to be the primary carrier protein of both 25(OH)D and 1,25(OH)2D.31 The 25(OH)D measured clinically reflects levels bound to VDBG and albumin with over 90% of the 25(OH)D bound to VDBG. Thus, in the setting of cirrhosis where VDBG production by hepatocytes is impaired, low 25(OH)D levels may not necessarily reflect vitamin D status. The strong correlation observed between serum levels of 25(OH)D and VDBG complicates assessment of vitamin D status in patients with advanced liver disease. We noted that VDBG was strongly correlated with total 25(OH)D levels but not with free 25(OH)D. In addition, VDBG showed only a moderate correlation with bioavailable 25(OH)D levels. In addition, only free and bioavailable 25(OH)D levels were significantly associated with albumin corrected serum calcium levels in these patients with cirrhosis. While more studies are needed to determine the optimal measures of vitamin D status in adults with advanced liver disease, the findings from this study and others19,20,32,33 suggest that calculating or measuring free or bioavailable 25(OH)D may provide additional information regarding vitamin D status in adults with liver disease, especially when disease is severe and VDBG levels are consequently low. We noted no association of vitamin D measures with iPTH levels consistent with previous studies.34–36 Among adults with non-cholestatic liver disease, iPTH levels are usually normal or even low despite low vitamin D levels reflecting the multiplicity of factors that influence bone turnover in the setting of liver disease including alcohol use, estrogen and testosterone levels and malnutrition.35
Our study has several limitations. First, the sample size was small and we selected patients across a spectrum of liver disease severity. Bioavailable and free 25(OH)D levels were calculated and not directly measured and calculated values may overestimate circulating free 25(OH)D levels.19 This study is also cross-sectional and cannot examine changes in vitamin D or VDBG levels as liver disease progresses. However, one previous study has shown that VDBG levels increase after liver transplantation.4 This study used an LC-MS/MS methodology to measure VDBG levels and this method avoids issues regarding the highly polymorphic nature of VDBG.(21) Assays that utilize antibodies directed against regions of VDBG that are polymorphic may yield erroneous values depending on the genotype. We used a method developed by Henderson, et al. that is independent of the VDBG isoform.21 In addition, calibration of vitamin D was verified using a NIST standard.
In summary, we show that VDBG levels correlate with liver disease severity. Levels of VDBG are also associated with total 25(OH)D and account for a substantial proportion of total 25(OH)D levels in adults with cirrhosis. In contrast, free and bioavailable 25(OH)D levels may not be influenced by VDBG levels, are associated with serum calcium levels and may be a useful alternative measure of vitamin D status in adults with liver disease. More studies are needed to determine the optimal measure of vitamin D status in adults with cirrhosis and low levels of VDBG.
The authors thank the participants of the liver disease cohort study conducted at Loyola University Chicago Medical Center. The findings from this study were presented in a poster at the Endocrine Society Meeting March 18, 2018 in Chicago, IL (DS).
HK and DS developed study design, analyzed data and co-wrote manuscript. RD assisted with study design, data analysis and manuscript revisions; PC assisted with data analysis and manuscript revision, SC enrolled participants in study, obtained serum specimens, and assisted with manuscript revisions. AH assisted with manuscript revision and interpretation of the data.
Internal funding mechanisms through Loyola University Medical Center.
Authors have no conflicts of interest to report.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.