eISSN: 2473-0815


The use of progesterone to prevent preterm delivery has been related to the increased risk of gestational diabetes (GD) development. We have shown previously that PG is able to trigger β-cells death and estrogens potentiated this progesterone effect. Since the expression of progesterone receptors (PR) is under control of estrogens in some tissues, we hypothesized that these hormones could regulate PR expression in pancreatic β-cells. The aim of this work was to evaluate the effect of estriol and estradiol on the regulation of the PRB (a physiologically active cytosolic receptor) and of PGRMC1 (the membrane component 1) expression in insulin-producing RINm5F cells. Cells were treated with the steroids hormones (estriol and 17b-estradiol) in pregnancy physiological or pharmacological concentrations for 6 or 24 h. RNA were extracted by TRIzol® method. Gene expression was analyzed by quantitative PCR using specific primers for PRB and PGRMC1. Treatment of cells with estriol or estradiol by 6 h slightly affected PGRMC1 expression. In contrast, 0.1, 1.0 and 10 μM estriol treatment for 6 h increased PRB expression by 15, 28 and 8-fold, respectively in comparison to untreated cells. Expression of PR was less affected after incubation of RINm5F cells for 24 h with the estrogens. These results confirmed that estrogens can regulate the expression of PR also in pancreatic cells, which may represent a mechanism to modulate progesterone action in these cells. The presented data may contribute to the better understanding of the interaction between steroid hormones and insulin producing cells, opening perspectives to the future treatment and therapeutic strategies for the GD management.
Keywords: progesterone, estrogens, progesterone receptors, b-cells, gestational diabetes
Ct, crossing threshold; E, estriol; ETD, estradiol; GD, gestational diabetes, GC, gene of control; GI, gene of interest; HPRT, phosphoribosyltransferase; PBS, phosphate-buffered saline; PI, propidium iodide; PG, progesterone; PR, progesterone receptor; PGRMC1, progesterone receptor membrane component 1; PRA, progesterone receptor isoform A; PRB, progesterone receptor isoform B; TNF- a, tumor necrosis factor alpha
Among the complications in pregnancy, gestational diabetes (GD) is one of the most common, affecting about 3-7% of pregnant women.1 GD is a condition characterized by intolerance to carbohydrates resulting in hyperglycemia, which may persist after delivery.2 According to the study of Hyperglycemia and Adverse Pregnancy Outcome3 and the International Diabetes Federation,4 pregnant women with GD, when untreated, have increased risk of premature rupture of membranes and preterm delivery. The fetus, on the other hand, has an increased chance to develop respiratory distress syndrome, cardiomyopathy, polycythemia, hypocalcemia and hypomagnesemia, hypoglycemia and macrosomia.
The GD pathophysiology is still unknown, but many studies have focused on the understanding of the action of specific hormones in pregnancy and its relationship to changes in carbohydrate metabolism during this period.5,6 Regardless, it has been shown that progesterone, whose high levels in the second trimester overlaps with GD onset, could contribute to insufficient insulin secretion to the increased demands of pregnancy.1 High progesterone levels have been also correlated with the development of glucose abnormalities in pregnancy and progesterone receptor-knockout mice are found to have improved glucose tolerance.7
Accordingly, Rebarber et al.,8 studied the incidence of GD among pregnant women who have received weekly doses of 250 mg progesterone intramuscularly for preterm delivery prevention. The results showed that the incidence of DG was higher inwomen who received progesterone (12.9%) compared to the control group (4.9%). Many studies, in contrast, have suggested a possible antidiabetic effect of estrogens. Le May et al.,9 demonstrated that estradiol, through the binding to ERα receptor, protected β-cells from apoptosis induced by oxidative damage. Another report showed that mice treated with estradiol showed β-cell hypertrophy, preventing diabetes development.10
The molecular mechanism involved in the progesterone action in DG scenario has not yet been clarified, however we have shown that progesterone is able to induce pancreatic β-cell death by an oxidative stress-dependent mechanism.11 Although estradiol and estriol have been related to cell protection from oxidative stress induced apoptosis, these hormones potentiated progesterone effect on β-cell death when used in pharmacological concentrations, even though they were proved to be much less toxic than progesterone.
In humans, the effects of progesterone are mediated by two distinct forms of the progesterone receptor (PR). They are transcribed from a single gene by alternate initiation of transcription from two distinct promoters,12 giving rise to transcripts that encode two protein isoforms, PRA and PRB. These proteins are identical in sequence, except that PRA lacks 164 amino acids at the N-terminus, making it shorter than PRB.13 There is evidence that both isoforms are functionally distinct. PRB is a strong activator of target genes, while PRA is a dominant repressor of the form B. Consequently, PRA high expression might result in a decreased responsiveness to progesterone.14
PR proteins are expressed in a variety of human tissues, including the uterus, mammary gland, brain, pancreas, bone, ovary and testis.15–20 The abundant expression of PR emphasizes the general physiological effects that progesterone can exert in a variety of organs throughout the body. Specifically, in β-cells the presence of PR in normal islets ranged from 5 to 20%, which suggest that progesterone may have a regulatory effect on the functional activity of the endocrine pancreas.21
PR expression and therefore the sensitivity to progesterone are under estrogens control. During pregnancy, plasma concentration of estradiol, the most potent estrogen, increases about 100 times, and of estriol about 1000 times compared to the pre-pregnancy levels.22 These hormones are able to increase PR expression in most target tissues, while progesterone decreases. Accordingly, it has been shown that the amount of PR protein is increased during proestrus or with the administration of exogenous estrogens in the mammalian uterus23 and other animal models.24,25 Corroborating this hypothesis, Bryś et al.,26 investigated the influence of administration of estriol for 14 days on the expression of PR in the endometrium, myometrium and vagina. Results showed that in women treated with estriol there was a significant increase in the expression of PR in the endometrium in comparison to control group.
Regarding to non-genomic mechanisms, progesterone membrane receptors have been also recently described in vertebrates.27 The expression of these receptors has been identified in tissues where the action of progesterone is less understood, including β-cells.28 Among these, the progesterone receptor membrane component 1 (PGRMC1) has been identified as an important novel mediator of progesterone function, being demonstrated that PGRMC1 is differentially expressed in fetal membranes among pregnant women and it is diminished in preterm delivery.29 Wang et al.,30 reported that the decrease in PGRMC1 expression and its translocation from a perinuclear location could contribute to a functional progesterone withdrawal that may ultimately initiate parturition. However, little is known about this receptor role in pancreatic tissue, and specifically in b-cells.
As progesterone effect on β-cell death was previously shown to be enhanced by estrogens, we showed here that estriol - the most prevalent estrogen in pregnancy -, and estradiol modulate the expression of PRB and PGRMC1 in insulin secreting RINm5F cells. These results should contribute to a better understanding of the DG pathophysiology, opening perspectives for the development of hormonal-based strategies for prevention and treatment of this disease.
Cell culture
Insulin producing RINm5F cells (a cell lineage derived from rat insulinoma) were maintained in RPMI-1640 supplemented with 24 mM sodium bicarbonate, 2 mM glutamine, 20 mM HEPES, 10 U/ml penicillin and 10 mg/ml streptomycin, 10% fetal bovine serum under humidified atmosphere at 37°C and 5% CO2. MCF7 and MDA-MB-231 breast cancer cell lines were used as positive and negative controls for PR expression, respectively. These cell lines were cultivated in the same coditions.
Progesterone and estrogens toxicity assay on RINm5F cells
To confirm the toxicity of progesterone and estrogens on RINm5F cells, experiments to evaluate cell membrane integrity loss and DNA fragmentation were performed. RINm5F cells were incubated with estriol, 17-β estradiol and progesterone (diluted in ethanol) in different concentrations (0.1, 1.0 and 10 μM), for 6 or 24 h. Progesterone and estriol were used at physiological (0.1 and 1.0 μM) and pharmacological concentrations (10 μM). Estradiol was used at the same concentrations, which are all pharmacological. After the incubation period, cells were collected by trypsinization and used in the different experiments.
Cell membrane integrity loss assay
Cells were centrifuged at 400 x g for 5 min at 4°C and the pellet was suspended in 500 μl phosphate-buffered saline (PBS). Subsequently, 5 μl of propidium iodide (PI) solution (1 mg/ml in PBS) were added and cells were analyzed using a Guava flow cytometer (Millipore Corporation, Hayward, CA, USA) according to Nicoletti et al.31 Fluorescence was measured using the FL2 channel (orange–red fluorescence 585/42 nm). A total of 10,000 events was analyzed per sample. Cells with PI fluorescence were evaluated using the in Cyte software (Millipore Corporation, Hayward, CA, USA).
DNA fragmentation assay
Apoptotic cells were evaluated by DNA fragmentation and loss of nuclear DNA content assay using the fluorochrome PI.31 After trypsinization, cells were centrifuged at 1,000 rpm for 5 min at 4°C. The pellet was gently added to 300 μl PBS containing 50 μg/ml PI, 0.1% sodium citrate and 0.1% Triton X-100. Cells were then analyzed by flow cytometry. A total of 10,000 events were analyzed per sample. Cells with PI fluorescence
were evaluated by using the in Cyte software (Millipore Corporation, Hayward, CA, USA).
Evaluation of progesterone receptors expression by quantitative PCR
Total RNA extraction
RINm5F cells were plated onto 6-well plates (105 cells per well) in a final volume of 2 ml and maintained in the culture conditions. After 48 h of plating, cells were treated with estriol or 17β-estradiol at the concentrations of 0.1, 1.0 and 10 μM for 6 or 24 h. MCF7 and MDA-MB-231 cells were treated with the same estriol or 17β-estradiol concentrations only for 6 h. Following these periods of incubation, cultures were washed with ice cold PBS and cells were lysed by the addition of 500 μl of TRIzolR (ThermoFisher). Cell lysates were immediately frozen at -80oC for RNA extraction.
After thawing, samples were incubated at 30°C for 5 min and added of 50 μL of chloroform. After brief vortexing and incubation at 30°C for 2 min, samples were centrifuged at 12,000 x g for 15 min. The aqueous phase was transferred to a sterile tube, which was added of 250 μL of isopropyl alcohol. Mixtures were incubated for 10 min at 30°C and thereafter centrifuged at 12,000 x g for 10 min. The supernatants were discarded and pellets were added of 500 μL of 70% ethanol. The samples were centrifuged at 7,500 x g for 15 min. Pellets were resuspended in 30 μL of ultrapure water. Samples were maintained at -80°C.
Complementary DNA obtaining
RNA samples were reverse transcribed with SuperScriptR III First-Strand Synthesis System, according to manufacturer’s recommendation (ThermoFisher). Briefly, for cDNA synthesis, 8 μl of RNA solution (2.5 μg) were mixed with Oligo dT18 50 μM (1.0 μl) and a mixture of 10 mM dNTPs (1.0 μl). Samples were incubated for 5 min at 65°C. Then, they were added of 10 x RT Buffer (2.0 μl), RnaseOUT 40 U/μl (1.0 μl), 25 mM MgCl2 (4.0 μl), 0.1 M DTT (2.0 μl), and Super Script R III RT 200U/μl (1.0 μl) in a final volume of 20 μl. Reverse transcription was carried out for 50 min at 50°C. The reaction was completed by heating at 85°C for 5 min. cDNA samples were kept at -20°C for the future experiments.
Primers designing
A set of primers were specifically designed for the progesterone receptors PRB and PGRMC1 cDNA as verified using BLAST (Basic Local Alignment Search Tool). Primer sequences are shown in the Table 1.
|
Sequence |
PRB F |
Forward: GCA TCG TCT GTA GTC TCG CCA ATA C |
PRB R |
Reverse: GCT CTG GGA TTT CTG CTT CTT CG |
PGRMC1 F |
Forward: ACT TCA CCC CTG CCG AAC TAA G |
PGRMC1 R |
Reverse: TCA TCC TTC AGT GCT TCT TTG TCC |
HPRT F |
Forward: CTC ATG GAC TGA TTA TGG ACA GGA C |
HPRT R |
Reverse: GCA GGT CAG CAA AGA ACT TAT AGC C |
Table 1 Primer sequences
Quantitative PCR (qPCR)
cDNA and primer optimal concentration were firstly determined. qPCR was performed using SYBR® Green PCR Master Mix (ThermoFisher). Briefly, 2.5 μl-aliquots of diluted cDNA (1:8) were mixed with the primers at a final concentration of 200 nM and 5 μl of Platinum SYBR® Master Mix (Invitrogen, Carlsbad, CA, USA). Reactions were developed with 40 thermal cycles of 95°C for 20 sec, 56oC for 30 sec, and 72°C for 55 sec using the Eco™ Real-Time PCR System (Illumina, San Diego, CA, USA). The cycle in which the reaction crosses the threshold (Ct) of the gene of interest (GI) was correlated with the amount of target mRNA. The hypoxanthine phosphoribosyltransferase (HPRT) gene was used as control (GC). The value of CtGC was subtracted from the value of the CtGI, resulting in ΔCt value, which represents the relative amount of the GI transcript. The increasing in gene expression was calculated as 2-ΔΔCt and gene expression was presented in relation to the control (cells treated with ethanol–the vehicle for progesterone dilution).
Statistical analysis
Cell viability and DNA fragmentation data were analyzed by one-way ANOVA plus Tukey's test for multiple comparisons. Gene expression data were analyzed by two-way ANOVA plus Bonfferroni post-test. Differences were considered significant for r<0.05.
Toxicity assay of progesterone and estrogen
Cultures were incubated with progesterone, estriol or estradiol at different concentrations for 6 or 24 h. As showed in the Figure 1, in the tested concentrations, these steroid hormones did not drive cells to necrosis, which was evaluated by cell membrane integrity loss.
The effect of steroid hormones on RINm5F cells was also evaluated by DNA fragmentation analysis. The data showed that 10 μM progesterone, when incubate for 24 h with the cells, caused a significant DNA fragmentation in comparison to the treatment for 6 h, in approximately 20% of cells (Figure 2). Also, 0.1 μM estradiol triggered DNA fragmentation in more than 25% of cells, whereas 1.0 and 10 μM estradiol in almost 60% of cells in comparison to cells incubated with ethanol (control). Estriol was the only steroid that did not promote DNA fragmentation in RINm5F cells in any concentration or time of incubation.
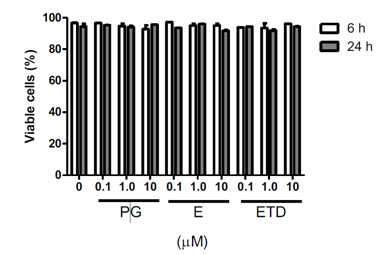
Figure 1 Effect of progesterone and estrogens on cell viability. Cells were incubated with the hormones at different concentrations for 6 or 24 h. Cell viability was evaluated, by flow cytometry, by the loss of plasma membrane integrity using PI. Data are presented as mean } SEM of three experiments in duplicates. Differences were analyzed by ANOVA + Bonferroni’s test and considered significant at ρ<0.05. Progesterone, E: Estriol, ETD: Estradiol.
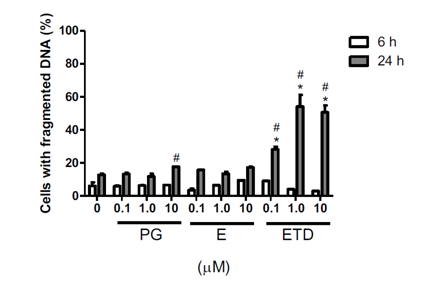
Figure 2 Effect of progesterone and estrogens on DNA fragmentation of RINm5F cells. Cells were incubated with the hormones, at different concentrations for 6 or 24 h. Data are presented as mean } SEM of three individual experiments in duplicates. Differences were analyzed by ANOVA + Bonferroni’s test and considered significant at p<0.05. (*) statistically different from untreated cells for the same time point; (#) differs from 6 h for the same treatment. PG: Progesterone, E: Estriol, ETD: Estradiol.
Progesterone receptors expression
It was analyzed the expression of PRB and PGRMC1, by quantitative PCR, after estrogens treatment. Estriol at the concentrations of 0.1, 1.0 and 10 μM modulated PRB expression in 15, 28 and 8-fold, respectively, after 6 h of incubation with RINm5F cells in comparison to control (Figure 3A). The maximum effect on PRB expression was in the incubation with 1.0 μM estriol, which corresponds to a pregnancy physiological concentration. This hormone did not cause significant changes in PRB expression when incubated for 24 h. Similarly, 17β-estradiol had a minor effect on PRB expression in RINm5F cells in all tested concentrations and in both time points. As expected, PRB expression was not verified or regulated by estriol or estradiol in MDA-MB-231 cells (Figure 3B), but it was observed an increase in PRB mRNA in MCF7 cells upon estriol treatment by 6 h. This hormone at the concentrations of 0.1 and 1.0 μM up regulated PRB expression by approximately 6 and 4-fold in comparison to cells incubated with only the vehicle (ethanol). Estradiol did not change PRB expression in MCF7 cell line in all tested conditions (Figure 3B).
PGRMC1 expression in RINm5F cells was slighter modified by estriol and estradiol treatments for 6 or 24 h (Figure 4). Estriol at a final concentration of 10 μM caused an enhancement of 4-fold in the expression of PGRMC1. Similar to PRB results, estriol showed an early effect in comparison to incubation for 24 h. Estradiol, in contrast, did not show a significant effect on PGRMC1 expression in both time points.
Figure 3 PRB expression evaluations by quantitative PCR. A) RINm5F or B) MDA-MD-231 and MCF7 cells were treated with estriol or 17b-estradiol in physiological (0.1 μM) or pharmacological (1.0 and 10 μM) concentrations for 6 or 24 h. After, cells were collected by trypsinization and total RNA was extracted by TRIzol® method. Expression was analyzed by quantitative PCR. HPRT was used as internal control gene. Results are shown as means + SEM of three experiments performed in duplicates. S/C: ratio Sample/Control. (*) statistically different from untreated cells for the same time point; (#) differs from 6 h for the same treatment. E: Estriol, ETD: Estradiol.
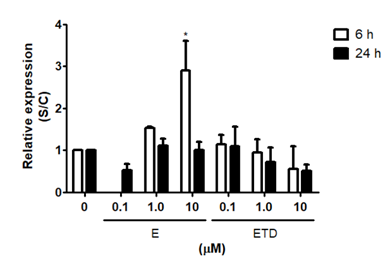
Figure 4 PGRMC1 expression evaluations by quantitative PCR. Cells were treated with estriol or estradiol in physiological (0.1 μM) or pharmacological (1 and 10 μM) concentrations for 6 and 24 h. After, cells were collected by trypsinization and total RNA was extracted by TRIzol® method. Expression was analyzed by real time PCR. HPRT was used as internal control gene. Results are shown as means + SEM of three experiments performed in duplicates. S/C: ratio Sample/Control. (*) statistically different from untreated cells for the same time point. E: Estriol, ETD: Estradiol.
The steroid hormones, progesterone and estrogens, have been implicated in the β-cell physiology exerting direct effects on insulin secretion21 and islet-cell proliferation9 through the binding to their specific receptors.32 Furthermore, Picard et al.,33 suggested that the hormonal particularities of pregnancy, and more specifically the progesterone, might contribute to the poor adaptation of insulin secretion21 to the increased requirements during pregnancy.
In contrast to the progesterone effects, data from human and animal studies indicated that 17β-estradiol prevents a decrease in insulin production in diabetic states.9 Also, estradiol, in pharmacological concentrations, protected human pancreatic islets from apoptosis induced by proinflammatory cytokines in vitro,34 suggesting that the antidiabetic action of estradiol might be due, at the least in part, to the β-cell apoptosis prevention.
We have already demonstrated that estriol, the main estrogen produced in pregnancy, was shown to be much less toxic to RINm5F cells than progesterone,11 but this hormone, at the pharmacological concentrations of 10 and 25 μM, caused a significant reduction of cell viability after 48 h of incubation. Also, the coincubation of cells with 25 μM estriol and 100 μM progesterone enhanced the loss of cell membrane integrity in both time points (24 and 48 h) in comparison to the cultures incubated with the hormones individually.
Few studies have reported the effect of estriol and estradiol on the expression of PR in b-cells, but since estriol potentiated progesterone-induced RINm5F cell death,11 and some authors have found that estradiol administration induced PR expression in various tissues, including pancreas,35 we hypothesized that these estrogens could regulate the expression of the progesterone receptors - PRB and PGRMC1-in b-cells, making these cells more sensitive to the progesterone action. Supporting this idea, Zhou et al.,36 found that progesterone-induced Min6 cell apoptosis was reduced in the presence of the selective PR antagonist SC51089.
We found that estriol modulated PRB expression, being the maximum effect (30- fold increasing) observed with 1.0 μM estriol, which corresponds to the pregnancy physiological concentration. On the other hand, 17β-estradiol was not able to modulate PRB expression in RINm5F cells in both physiological and pharmacological concentrations, suggesting that the progesterone effect upon RINm5F cells is not dependent on this hormone directly.
Similarly, Diller et al.,37 showed that in MCF-7 cells, a cell lineage derived from ductal invasive carcinoma, estriol in a nanomolar range, upregulated PR expression, which was accompanied by an increase in the protein level of the receptor. Corroborating our results, Zheng & Lin 38 showed that estradiol did not present any effect on PR
expression after 72 h of incubation with MCF-7 cells, mostly due to the complete abolishment of the estrogenic activity by the metabolic degradation of the hormone up to 80% over 24 h.
In addition to the investigation of the typical progesterone receptor PRB expression, we evaluated the modulation of PGRMC1, which is a progesterone receptor membrane component. We have shown that PGRMC1 expression in RINm5F cells was only lesser modified by estriol or estradiol treatments, even this receptor seems to be very expressed in β-cells in terms of mRNA (data not shown). Estriol at a final concentration of 10 μM caused an enhancement of 4-fold whereas estradiol did not present a significant effect on PGRMC1 expression in both time points. This result suggests that PGRMC1 expression is not directly affected by estradiol but can be modulated by estriol in a pharmacological concentration.
Originally, PGRMC1 has been investigated in ovarian and breast cancers, where progesterone exhibits an antiapoptotic activity. Nevertheless, PGRMC1-depleted cells exhibited apoptotic phenotype when treated with progesterone,39 suggesting a potential role of this receptor as a marker for neoplasia.40
Little is known about the PGRMC1 function in insulin producing cells, as well as and in pregnancy and gestational diabetes scenario, but it has been shown that this receptor is expressed in human placenta, primarily in syncytiotrophoblasts, smooth muscle cells of the placental vasculature, and in villous capillaries during the pregnancy.41
The function of PGRMC1 in preterm delivery prevention by progesterone treatment has been also studied. Despite the molecular mechanisms are not completely understood, it has been shown that progesterone and medroxyprogesterone 17-(MPA) inhibit apoptosis induced by TNF-α in fetal membranes, an event that combined to the extracellular matrix degradation by a matrix metalloproteinase (MMP-9) contribute to premature delivery.42 These authors showed that the binding of MPA to PGRMC1 decreases the expression of MMP-9, which corroborate the idea about the use of this hormone in preterm delivery prevention.
Additionally, Bali et al.,43 studied PGRMC1 modulation by steroid hormones in hippocampal regions of ovariectomized rats. Treatment with progesterone and estradiol for 4 or 30 days caused upregulation in PGRMC1 expression. According to Peluso et al.,44 the effect of progesterone binding to the PGRMC1 is mediated by a mechanism dependent of protein kinase G and the interaction with other binders such as the mRNA binding protein, serpin 1. 45 However, there is still no direct evidence of progesterone binding to PGRMC1.46
The function of PR in β-cells and its modulation by estrogens is still unclear, however, based on our results we suggest that the increasing in PRB expression induced by estriol could be associated to the responsiveness of these cells to progesterone action,11 even we did not examine the PRB expression in protein level. We also demonstrated that PGRMC1, which is very well expressed in β-cells, was only slightly modulated by estrogens, indicating that, at least in these cells, other mechanisms and molecules are responsible for the regulation of PGRMC1 expression. Together, the data presented here should contribute to a better understanding of the pancreatic β-cell biology during pregnancy and GD pathophysiology, opening perspectives for different approaches and
hormone-based therapeutic strategies for GD management.
In summary, we have shown that estriol increases the expression of PRB in terms of mRNA. Considering that protein expression was correlated with RNA expression, we suggest that this result could explain the potentiation of progesterone toxic effect upon insulin producing RINm5F cell death.
This work was supported by Fundacao de Amparo a Pesquisa do Estado de Sao Paulo–FAPESP (2016/14150-2).
We state that there is any financial or other potential conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.