eISSN: 2473-0815


Aim: Rapid lowering of blood glucose has been suggested to accelerate the progression of retinopathy in pregnant diabetic women. We examined the relationship between the degree of glycemic control or the fluctuations in blood glucose and the course of retinopathy in pregnant women with type 1 diabetes mellitus.
Methods: Retrospective analysis of glycemic parameters and severity of retinopathy of 49 sets of two trimesters in 38 women with type 1 diabetes mellitus documented with seven standard field stereo fundus photographs.
Results: Retinopathy progressed in 18(36.7 %) sets of two trimesters. Age, duration of diabetes, White classification, hypertension and gravidity were not significantly correlated with changes in severity of retinopathy. Base-line HbA1c was significantly higher in the progression group (P=0.003). The magnitude of the decreased in HbAc1 from base line was greater in the progression group (P=0.04). Logistic regression model was used to determine the influence of several risk factors in describing the change in severity of retinopathy between visits. Base line higher HbA1c was the only significant variable in describing progression (P=0.01). Base line higher HbA1c was associated significantly with the development of soft exudates (P=0.02) and soft exudate/IRMA (P=0.01). No statistically difference was found between base line means, standard deviation of the means of the blood glucose levels of each five measurements during the day or the standard deviation of all of the blood glucose levels in the groups and individual lesions analysis.
Conclusion: Poor glycemic control early in pregnancy and the magnitude of improvement in glycemic control rather than the fluctuations in the blood glucose levels may be critical in the progression in diabetic retinopathy.
Keywords: type 1 diabetes mellitus, retinopathy, proliferative diabetic retinopathy
ETDRS, early treatment diabetes retinopathy scale; SES, soft exudates; PDR, proliferative diabetic retinopathy
Although evidences have shown that long term near normal glycemic control delays the progression of microvascular complications in type 1 diabetes including retinopathy,1–3 rapid near normalization of glycemic control has been suggested to accelerate the progression of retinopathy in nonpregnant4–8 and pregnant9–12 diabetic patients. Progression of diabetic retinopathy during pregnancy could occur in 14-85% of cases,9,11–22 others found no association.23–25 Study on normal cat found that the retinovascular response to glucose infusion suggests a breakdown in the normal response of tissue autoregulation.26 Although elevated blood glucose levels are associated with decreased retinal arterial regulatory responses which could play a role in the development of diabetic retinopathy,27,28 gradual decrease in blood glucose may be beneficial.29 Short term fluctuations in plasma glucose levels were not associated with hemodynamic changes in the macular capillaries in patients with established retinopathy.30 To clarify this issue and explore the etiology of transient worsening of retinopathy, we addressed:
We conducted a retrospective analysis of 49 sets of two trimesters in 38 women who completed 41 pregnancies under strict blood glucose control with type 1diabetes who were followed in the diabetes clinic at the British Columbia Women’s Hospital and the University of British Columbia Ocular Diabetes Clinic. All eligible women, defined as having a diagnosis of type 1 diabetes using standard clinical criteria31and at least two visits to both clinics during the pregnancy, are included in this report. Data were obtained from chart review and contact with women and their physician. The ethical review board of the University of British Columbia approved the study. Complete medical and obstetric histories were documented in the chart. Pregnancy dating was based on menstrual dates and confirmed by ultrasonographic examination. Glycemic control was maintained with regimens of short- and intermediate-acting insulin preparations injected two to four times per day and insulin adjustment along with dietary regulation was made daily by a team of diabetes nurse clinicians and physicians. The diabetes clinic asked women to monitor blood glucose at least four times a day and aimed for glucose levels of 4-6 mmol/l before meals. Blood glucose levels of pre-meals including breakfast, lunch, supper, bedtime, and 2 am were measured. HbA1c values were measured in clinical laboratories with the normal range 4.3-6.2, value expressed as time the power of 10-2. White classes for diabetes in pregnancy are defined as B: onset after age 20 and duration less than 10 years, C: onset between ages 10 and 19 or duration of 10-19 years, D: onset before age 10 or duration more than 20 years or hypertension, R: proliferative retinopathy, F: nephropathy, RF: both proliferative retinopathy and nephropathy. Hypertension before pregnancy was diagnosed if the blood pressure raised more than 140/90 or on anti-hypertensive medications. Current age was defined as the age in years as reported at the first visit. Duration of diabetes was the period between the reported age at diagnosis of diabetes and the age at the first visit.
Retinopathy assessment
Seven standard fields stereoscopic color fundus photographs were available for comparison for the two trimesters and graded (with grader blinded to the clinical data) according to the Early Treatment Diabetes Retinopathy Scale (ETDRS) modifications of the Airlie House classification which provides a grade for the severity of each type of lesion of diabetic retinopathy for each eye. Grades for the various lesions were used to derive overall retinopathy severity levels for each woman according to the ETDRS interim and final scale.32,33 Retinopathy outcomes were divided into two groups; progression and non-progression as compared with base line retinal assessment. Retinopathy progression was defined as a higher grade of retinopathy on the final ETDRS by at least one Step.34 The development of soft exudates (SEs), intraretinal microvascular abnormalities (IRMAs), and soft exudate/intraretinal microvascular abnormality were analyzed separately.35–37
Statistic analysis
The unit of analysis in this paper is a set of two trimesters. Mean, standard deviation and 95 % confidence intervals of the mean, the standard deviation of each five measurements of blood glucose levels during the day were measured for analysis. Data were analyzed with SPSS V 22.0 .Unpaired t-test student used for parametric and Mann-Whitney test for nonparametric data, chi square and Fisher’s exact used for categorical data. Logistic regression model was used to determine the influence of several risk factors in describing the change in severity of retinopathy between visits. P<0.05 is significant.
Of 300 hospital charts reviewed, 38 type 1 diabetic women were pregnant with 41 completed pregnancies and 49 sets of two trimesters in which seven standard field colour fundus photographs had been taken fulfilled the inclusion criteria were eligible for analysis. A woman ranged in age from 19 to 39 years (mean±standard deviation, 28.7±4.5).The duration of diabetes was 3 to 31 years. The body mass index was 19.4 to 35.5kg/m (mean±standard deviation, 24.4±3.4). As shown in Table 1, progression group has longer diabetes duration (P = 0.2). White class B was found in 22.6 % of the nonprogression group (Odd ratio=5.0 (0.6 - 44.1), P=0.2), where as Class C (Odd ratio=1.6 (0.4 – 6.3), P=0.5) and D-ER-F (Odd ratio=1.4 (0.4 -4.9), P=0.6) were associated nonsignificantly with progression. Because these cases were compared in different trimesters of pregnancy, we found, although the numbers are small, there was 3.8-fold (1.1–13.1, P=0.03) significant association with retinopathy not to be progressed if the baseline retinal assessment were at the second trimester which may not suggest a possible peak during the second trimester as it was suggested.22 There was 2.9-fold (0.8-9.8) nonsignificant increase risk of progression in the first trimester (50 % vs. 25.8 %, P=0.09). Age, BMI, chronic hypertension, pregnancy induced hypertension, gravidity, and gestational age were not significantly associated with changes of retinopathy between visits.
Retinopathy status as listed in Table 1, 18 (36.7 %) compared trimesters were progressed, 31(63.3 %) showed no progression from baseline. At baseline, no retinopathy was found in 2 sets of trimesters (P=0.7). Microaneurysm or hemorrhage without microaneurysm were progressed in 38.9 % (Odd ratio=1.8(0.5–6.3), P=0.3). Mild nonproliferative diabetic retinopathy was found in 16.7 % in the progression group (Odd ratio=0.5 (0.1–2.1), P=0.5). Moderate nonproliferative diabetic retinopathy was present in 38.9 % in the progression group, 9.7 % in the nonprogression group (Odd ratio=5.9 (1.3–27.2), P=0.025). At baseline, the non-progression group showed moderately severe nonproliferative diabetic retinopathy (P=0.5) and proliferative diabetic retinopathy (PDR) (P=0.1) in 6.5 % and 25.8 % of the sets of trimesters respectively. 55.6 % were progressed by one step. Soft exudates were developed in 22.4 %, where micro-aneurysm or hemorrhage without micro-aneurysm significantly found at base line (odds ratio=5.5 (1.2-25), P=0.03). The presence of IRMA in 16.3 % which was significantly associated with presence of moderate NPDR at baseline (odds ratio=32.5 (4.3-244.2), P=0.001). No significant correlations between SEs / IRMAs with any base line retinopathy grade.
Parameters |
Retinopathy outcomes |
P value |
|
|
Progression |
Non-progression |
|
Total |
18(36.7) |
31(63.3) |
|
Age (years) |
29.1±4.4 |
28.5±4.7 |
0.6 |
Body mass index (kg/m) |
23.9±3.8 |
24.7±3.2 |
0.4 |
Diabetes duration (years) |
17(13.8, 25) |
15.5(9.5, 21) |
0.2 |
White Classification |
|||
B |
1(5.6) |
7(22.6) |
0.2 |
C |
5(27.8) |
6(19.4) |
0.5 |
D-RF |
12(66.7) |
18(58.1) |
0.6 |
Chronic hypertension |
2(11.1) |
1(3.2) |
0.5 |
Pregnancy induced hypertension |
8(44.4) |
7(22.6) |
0.1 |
Primigravida |
8(44.4) |
10(32.3) |
0.5 |
Gestational age (days) |
259.3±8 |
255.2±8.9 |
0.1 |
Birth weight (kg) |
3.8±0.6 |
3.6±0.7 |
0.2 |
Time point of trimesters |
|||
1st trimester-2nd trimester |
9(50) |
8(25.8) |
0.09 |
2nd trimester-3rd trimester |
7(38.9) |
22(71) |
0.03 |
1st trimester-3rd trimester |
2(11.1) |
1(3.2) |
0.5 |
Retinopathy |
|||
No retinopathy |
1(5.6) |
1(3.2) |
0.7 |
Microaneurysm or hemorrhage |
7(38.9) |
8(25.8) |
0.3 |
Mild nonproliferative diabetic retinopathy |
3(16.7) |
9(29) |
0.5 |
Moderate nonproliferative diabetic retinopathy |
7(38.9) |
3(9.7) |
0.03 |
Moderately severe nonproliferative diabetic retinopathy |
0 |
2(6.5) |
0.5 |
Proliferative diabetic retinopathy |
0 |
8(25.8) |
0.1 |
Table 1 Characteristics of retinopathy outcomes at baseline
Data are number (%), mean (SD), or median (25th centile, 75th centile)
Glycemic control
A comparison of the severity of the retinopathy with the metabolic parameters is shown in Table 2. Progression group had higher base-line HbA1c (P=0.003). The magnitude of HbA1C decreased from base-line was higher significantly in the progression group (P=0.04), with significant 4.5-fold (1.0–19.1) increase in the risk of progression if the magnitude decreased in the HbA1c concentration by 1.5 or more (P=0.04, Chi square test). We looked at the severity of retinopathy between the two visits by HbA1c quartiles (Table 3). In the first quartile of HbA1c (<5.7), the sets of trimesters had a greater tendency not to progress, P=0.003), while in the fourth quartile of the HbA1c (>7.7); the sets of trimesters had a greater tendency to progress (odds ratio=5.0 (1.2-20.5), P=0.04). Logistic regression model was used to determine the influence of several risk factors in describing the changes in severity of retinopathy between visits. Base line higher HbA1c was the only significant variable in describing progression (P=0.01). The mean blood glucose levels and the standard deviations of the means of the five measurements during the day were not statistically different between the two groups. The standard deviation of the absolute blood glucose values of the five measurements during the day were not statistically different (Figure 1 & Figure 2). As shown in Table 4, base line higher HbA1c was associated with the development of soft exudates (P=0.02) and soft exudates/IRMAs (P=0.01). The magnitude of decrease in HbA1c was associated with the development of soft exudates, with 5.8–fold (1.2-28.4) increase in the risk of development of soft exudates if the magnitude of decrease in HbA1c concentration was 1.5 or more (P = 0.03, fisher’s exact test) and soft exudates/IRMA (P=0.02) with 4.5-fold (1.0-19.1) increase risk of development if magnitude decreased of HbA1c concentration was 1.5 or more (P=0.04, chi square test). There were no statistically different between the base line HbA1c, the magnitude of decrease in HbA1c from base line, means, standard deviation of the means of the blood glucose levels of the five measurements during the day and the standard deviation of the each five measurements of daily blood glucose levels in the analyzed individual lesions.
HbA1c |
1st Quartile |
2nd Quartile |
3rd Quartile |
4th Quartile |
Progression |
0 |
4(40) |
6(42.9) |
8 (66.7) |
Non-progression |
11(100) |
6(60) |
8(57.1) |
4 (33.3) |
Odds ratio (95% confidence intervals) |
1.6(1.2-2.1) |
1.1(0.3-4.6) |
1.3(0.44.7) |
5(1.220.4) |
P value |
0.003 |
0.9 |
0.7 |
0.04 |
Table 2 HbA1c of retinopathy outcomes at baseline and follow up
Data are mean±SD ( 95% Confidence intervals )
HbA1c |
1st Quartile |
2nd Quartile |
3rd Quartile |
4th Quartile |
Progression |
0 |
4(40) |
6(42.9) |
8(66.7) |
Non-progression |
11(100) |
6(60) |
8(57.1) |
4(33.3) |
Odds ratio (95% confidence intervals) |
1.6(1.2-2.1) |
1.1(0.3-4.6) |
1.3(0.44.7) |
5(1.220.4) |
P value |
0.003 |
0.9 |
0.7 |
0.04 |
Table 3 Retinopathy outcomes and HbA1c quartiles at baseline
Data are n(%)
Parameters |
Retinopathy lesions |
HbA1c at Baseline |
HbA1c at Follow up |
Decrease in HbA1c |
Soft exudates |
Present |
7.3±1.1(6.5-8.0) |
5.7±0.8 (5.1-6.4) |
1.5±0.9 (0.8-2.2) |
Absent |
6.4 ±1.1 (6.0 -6.8) |
5.8 ± 0.9 (5.5-6.1) |
0.7±0.8(0.4-1.0) |
|
P value |
0.023 |
0.9 |
0.013 |
|
IRMA |
Present |
6.8±0.8(6.1-7.5) |
5.9±0.7(5.3-6.5) |
0.9±0.8(0.1-1.6 ) |
Absent |
6.6±1.2(6.2-6.9) |
5.7±0.9(5.4-6.1) |
0.8±0.8 (0.6-1.2) |
|
P value |
0.6 |
0.6 |
0.9 |
|
SE / IRMA |
Present |
7.1±1.0 (6.6-7.6) |
5.8±0.8(5.4-6.2 ) |
1.3±0.9 (0.8-1.7) |
Absent |
6.3±1.1 (5.8-6.7) |
5.7±1.0(5.4-6.1) |
0.6±0.8 ( 0.3-1.0 ) |
|
P value |
0.01 |
0.8 |
0.024 |
|
Progression (steps) |
1 |
7.0±0.8 (6.5-7.6) |
6.0±0.9(5.3-6.7) |
1.0±0.9 ( 0.3-1.8 ) |
≥2 |
7.4±1.1 (6.5-8.3) |
6.0±0.7 (5.4-6.5) |
1.4±1.0 ( 0.6-2.3 ) |
|
|
P value |
0.4 |
0.9 |
0.4 |
Table 4 HbA1c at baseline and follow up in relation to the development of soft exudates, IRMAs, SE and/or IRMA, and progression by steps
Data are mean+/- SD (95 % Confidence intervals)
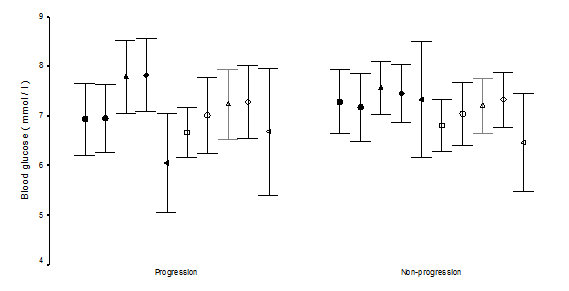
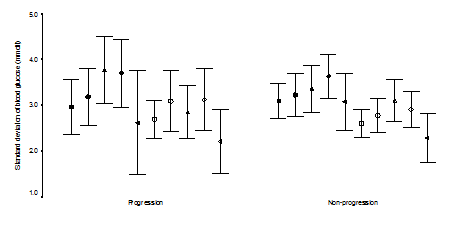
We have found retrospectively, diabetic retinopathy progressed in 36.7 %. Because we studied sets of two trimesters as a unit of analysis, we found a higher incidence of progression of 39 % in the 41 pregnancies studied, which falls within the 14-85 % incidence reported earlier,9,11–22 reflecting the value of serial ophthalmoloscopy and photography in pregnant type 1 diabetics as previously suggested.15 Dibblie et al.14 suggested that background retinopathy may wax and wane during pregnancy. Hellstedt et al.35 reported some of the microaneurysms are occluded temporarily by fibrin or platelets thrombi and then re-canalized, which may explain some of the micro-aneurysms disappearance in our nonprogression group, however, we found that nonprogression of the retinopathy was associated not significantly with mild diabetic retinopathy at base line (odds ratio 2.0 (0.5-8.8), P=0.49) but the number of cases (9 cases) are small. However, base line moderate NPDR was associated with 5.9-fold increase in the risk of progression (P=0.03). Understanding the risk factors that may lead to progression among diabetic women in pregnancy is of great important in the management of such cases. Therefore, we investigated the risk factors that have been proposed to influence the progression of diabetic retinopathy. Diabetes duration has been found to be correlated inversely with progression of retinopathy in non-pregnant38,39 and pregnant women.19,21 Duration of diabetes was not significantly associated with the progression group when compared to the nonprogression group (P=0.2) in contrast to the previous report,9 however, subgroup of the non-progression whom retinopathy were improved from baseline had shorter duration of diabetes (6 years) when compared to progression (Odd ratio=22.7 (1.8–279.4), P=0.01, fisher’s exact test) which may emphasize as suggested by Soubrane et al.17 the advantage of early planning pregnancy in this population. The pathogenesis of diabetic retinopathy has been the focus in earlier studies. In such way, they could explain our findings. The rate of oxygen consumption is higher38 and the oxygen released capacity is reduced in diabetic patients,39 and the reduced oxygen consumption of the retina found in hyperglycemia may worsen the relative hypoxia.40 The increased arterial retinal blood flow found in early diabetes,27,41,42 and in hyperglycemia26 may also be reduced leading to ischemia10 after rapid normalization of blood glucose. In contrast to earlier reports,21,43 we found progression of retinopathy was related to the degree of glycemic control achieved over the course of the two trimesters studied (P=0.04) which was found by Phleps, Klein & Rosenen et al.9,11,19 and the DCCT22 and in nonpregnant study.44 Although we found the HbA1c was significantly higher in the progression group at baseline (P=0.003) as previously found in the nonpregnant44 and pregnant diabetic,12,22 other found association was not significant.21 The correlation between baseline HbA1c concentration and the magnitude of decrease of HbA1c between the two visits was 0.631 (P< 0.01); higher baseline HbA1c concentration were associated with greater decreases in HbA1c between the two visits which was consistent with Phleps et al.9 Davis et al.30 suggested that short term fluctuations in plasma glucose levels are not associated with hemodynamic changes in the macular capillaries in patients who have established retinopathy in 20 non-pregnant poorly controlled type 1 diabetic patients. A mechanism has been suggested by Grunwald et al.29 in study of poorly controlled type 2 patients (diabetes duration was 7.3±5 years) by using laser doppler velocimetry which measures blood flow in the major retinal arteries and veins, that decrease in volume flow accompanying the insulin induced drop in blood glucose levels correlated significantly with the patient’s duration of diabetes. The shorter diabetes duration, the largest decrease in flow. The duration of diabetes between the group studied by Davies et al (18.5±7) and our progression group (18.2 ±6.7) were similar compared to the poorly controlled type 2 patients (7.3±5) studied by Grunwald et al.27 may suggest patients responses to lower plasma glucose levels would be small as in Davies et al patients by using the blue light entopic technique and more difficult to be detected clinically by us. Our data provide the first detailed retrospective evidence in pregnant type 1 diabetic women that no significant correlations were found between fluctuations in the daily blood glucose levels and the changes in diabetic retinopathy. Thus, we believe our data indicate that poor glycemic control early in pregnancy and the rapidity and magnitude of improvement in glycemic control rather than the fluctuation in the blood glucose levels may be critical in the progression in diabetic retinopathy, supporting the opinion of Chew et al.12 to emphasize the importance of preconception normalization of HbA1c to avoid the risk of progression in pregnant women with type 1 diabetes mellitus. Soft exudates (SE) and intraretinal microvascular Abnormalities (IRMAs) are changes encountered during pregnant9,10,15,18 or nonpregnant patients.1,5,6,44 The soft exudates represent microinfarction in the nerve fibre layer, whereas the nature of IRMAs is controversial.5 They were often regarded as characteristic of the preproliferative phase of diabetic retinopathy, and commonly preceding the appearance of new vessels.1 SEs developed in 22.4 % of the cases in our series, where as IRMAs developed in 16.3 % of the cases. In agreement with Dahl-Jorgensen et al.6 when SEs developed in the first months of treatment in his study, SEs developed during our comparison of trimester’s duration.
Institution of strict metabolic control associated with the development of SEs as shown by higher HbA1c concentration at baseline (P=0.02) and the magnitude of HbA1c change (P=0.01).5,6,9,44 These significant correlations were not found for the development of IRMAs (P=0.6). This may be explained by the relative good glycemic control at baseline and the drop in HbA1c concentration were not high. However, the higher HbA1c concentration at baseline (P=0.01) and decreased in the HbA1c concentration from baseline (P=0.02) may contribute in development of SEs / IRMAs.44 In contrast to the DCCT,22,44 as evidence by our data, steps progression were not significant correlated with the higher HbA1c concentration at baseline (P=0.43) and decreased in HbA1c concentration from baseline (P=0.4), that could be attributed to both groups start with similar HbA1c concentration and the magnitude of HbA1c change between the different in steps were identical and the sample size were small to show a significant difference. As the case earlier, the Plasma glucose fluctuation in the daily blood glucose levels was not correlated. To conclude; we believe our data indicate that poor glycemic control early in pregnancy and the rapidity and magnitude of improvement in glycemic control rather than the fluctuations in the blood glucose levels may be critical in the progression in diabetic retinopathy.
We are grateful to all who assisted in this study at the British Bolumbia women and children hospital, Vancouver, British Columbia Canada. No grants were assigned to this study.
Authors have no conflicts of interests.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.