eISSN: 2473-0815


There is evidence which supports the role of lipids in male and female infertility. Lipids are involved in basic molecular processes for reproduction, such as cholesterol, which is the main substrate for steroid synthesis and has been shown to affect the hormonal milieu and steroidogenesis in both men and women. However, its association with male and female infertility was still not clear. Association’s in-between lipid levels in seminal plasma with semen parameters and cholesterol levels in follicular fluid with ovarian reserve showed no clear correlation.
The aim of this study was to assess the total levels of cholesterol, triglycerides (TG) and non-esterified fatty acids (NEFA) in serum, seminal and follicular fluid of infertile men and women comparing with healthy controls.
Semen samples from 20 patients attending the Andrology Laboratory in Clinica Tambre with low sperm count and motility were included in this study. A control group was formed by 39 fertile men with normal semen parameters. Follicular fluid (FF) was obtained from 14patients <35years old, low responders (≤5oocytes retrieved) undergoing IVF (in vitro fertilisation) and 40 healthy fertile oocyte donors, both under the same ovarian stimulation protocol. The total levels of cholesterol, triglyceride (TG) and non-esterified fatty acids (NEFA) were determined by commercial kits. T-Student test was used to verify normality distribution of the variables. One-way-ANOVA was used to compare the differences among the three groups.
In the group of male patients, the levels of TG and NEFA in seminal fluids and TG in plasma were significantly elevated as compared to the control group. TG in follicular fluid and NEFA concentrations in serum and follicular fluid were significantly higher in patients with low response in comparison to the control group.
In conclusion, altered sperm parameters and low ovarian reserve are associated with elevated triglycerides and fatty acids in seminal plasma and ovarian follicular fluid. Gamete maturation within this lipid-rich environment is detrimental to spermatozoa and oocytes.
Keywords: male infertility, female infertility, lipids
TG: Triglycerides; NEFA: Non-Esterified Fatty Acids; FF: Follicular Fluid; HDL: High-Density Lipoprotein; LR: Low Responders
The role of lipids in male and female fertility is extensively supported in the literature.1‒8 Increased high-density lipoprotein (HDL) concentrations have been associated with better oocyte and embryo outcomes.3,4 as well as being correlated with beneficial effects on spermatogenesis.9,10 Hypothesis on the role of lipids in human reproduction have also arisen as cholesterol is the main substrate for steroid synthesis.1 which, in fact, has been shown to affect the hormonal milieu and steroidogenesis in both men and women.5
On the one hand, abnormal lipid metabolism is closely associated with spermatogenesis, sperm maturation and capacitation disorders when evaluating male subjects.11,12 On the other hand, regarding the female population, oocytes developing within ovarian follicles grow rapidly and require supply of energy and cholesterol.13 Oocytes are surrounded by follicular fluid (FF) that, in contrast to human plasma, mainly contains HDL cholesterol which is in fact the smallest lipoprotein subclass.14 Even though expression of the LDL receptor as well as the LDL receptor-related protein 4 has been reported in mammalian oocytes,15 most of the research has focused on HDL within FF.
The aim of this study was to assess the total levels of cholesterol, triglyceride (TG) and non-esterified fatty acids (NEFA) in serum, seminal and follicular fluid of infertile men and women comparing with healthy controls.
This prospective clinical study was conducted at Clinica Tambre from February 2017 to June 2017. It was approved by the Ethical Review Board of the Hospital de la Princesa (Madrid, Spain).
Male patients
Semen samples were obtained from 20patients (aged in between 28=46years old) classified as oligoasthenoteratozoospermic (OATz) according to WHO 2010 criteria, who attended the Andrology Laboratory in Clinica Tambre.16 Patients included in the study had to meet the following inclusion criteria: non-azoospermic patients with a normal 46, XY karyotype evaluated by conventional cytogenetic analysis, normal hormone profile, no history of radiotherapy, chemotherapy, chronic illness or medication, and patients without sperm defects genetically originated. Physical examination and scrotal Eco-color Doppler were performed on all patients to detect the presence of varicocele. To identify the presence of clinically asymptomatic genitourinary infections, a bacteriological analysis was performed on all semen samples. All infertile patients were individuals who did not obtain pregnancy after 2years of unprotected sexual intercourse.
The control group was formed by 39men with proven fertility as sperm donors in Clinica Tambre. Fertile men (aged in-between 24-40years old) with normal karyotype and semen parameters >25 percentile.16 and not affected by anatomical problems and/or infections. All patients gave informed consent for this research.
Semen analysis
Semen samples were collected by masturbation after a period of 3-5days of sexual abstinence. After liquefaction (37ºC, 30min), smears of neat semen were prepared for sperm morphology assessment and sperm concentration and motility were evaluated according to the WHO criteria.16
Female patients
Follicular fluids (FF) were obtained from 14patients <35years old, low responders (LR) (≤5 oocytes retrieved after follicular punction) undergoing IVF and 40 healthy fertile oocyte donors, both under the same ovarian stimulation protocol.
Patients were recruited according to the following criteria:
Women were included as donors after being thoroughly informed about oocyte donation and later fully evaluated to assess fulfillment of the criteria required to be admitted into a donation program. In short, oocyte donors were in between 18-35years old. Complete medical history examination was performed, which had to include absence of current or past exposure to radiation or hazardous chemical substances, drug abuse and past reproductive history. All donors must had had, as a result of the exhaustive evaluation, a normal physical and gynecological examination, BMI in between 19 and 26kg/m2, no family history of hereditary or chromosomal diseases, normal karyotype and negative screening for sexually transmitted diseases (STD).19
Ovarian stimulation protocols
Ovarian stimulation in patients was initiated with 225-300UI/day rec-FSH (Gonal-F®; Merck, Madrid, Spain) from day 2 of the menstrual cycle. The GnRH antagonist (Cetrotide; Merck, Madrid, Spain) was introduced according to a multiple-dose protocol (0.25mg/day) when a leading follicle of 14mm and/or oestradiol concentrations of 400pg/ml was reached. Recombinant human chorionic gonadotrophin (HCG) (Ovitrelle®, Merck, Madrid, Spain) was applied when ≥2 follicles reached ≥17mm and oocyte retrieval was performed under sedation at the 36th hour following HCG.
The ovarian stimulation in donors began with 125–225IU of recombinant FSH (Gonal-F®; Merck Serono, Madrid, Spain) from day 2 of the menstrual cycle. The GnRH antagonist (Cetrotide; Merck, Madrid, Spain) was introduced according to a multiple-dose protocol (0.25mg/day) when a leading follicle of 14mm and/or oestradiol concentration of 400pg/ml was reached. Triggering was performed when at least three follicles >17 mm were present with 0.2mg of triptorelin (SC Decapeptyl, Ipsen Pharma, Barcelona, Spain) and oocyte retrieval was performed under sedation at the 36th hour following GnRHa. In all groups, the first control (ultrasonography and serum oestradiol) was performed after 5days of stimulation, and the daily dose of FSH was adjusted individually according to the ovarian response.
Seminal, follicular and blood plasma sample preparation
Seminal plasma was obtained by centrifuging the semen samples at 300g for 7min at room temperature. Samples were stored in liquid nitrogen. At the time of semen retrieval, 5 ml of fasting blood from each male was placed in an EDTA-coated tube and centrifuged at 600g for 15min at 48C. For each analysis, 100ml of blood plasma was added to 0.9ml of BHA solution and stored in liquid nitrogen.
Oocytes were separated and placed into culture media, whereas follicular fluid was collected in flasks.20 Only uncontaminated follicular fluid minimally stained with blood were kept for further determinations. At oocyte retrieval, and after removing the oocytes, follicular aspirates were centrifuged at 600g for 10min and the supernatant stored at −70°C for a maximum of 2weeks.
Venous blood samples were placed in an EDTA-coated tube and centrifuged at 600g for 15min to isolate serum for detection of lipid level.
Total cholesterol, triglyceride (TG) and non-esterified fatty acids (NEFA) acid analysis
Lipids were separated according to the standard Folch method.21 Briefly, 100ml of sample (blood, follicular fluid or seminal plasma in BHA) was mixed with 1.9 ml of chloroform/methanol (2:1v/v) and 1ml of cold water. The mixture was vortexed for 90min at 48C. From the resulting organic phase, aliquots were then used for phospholipid analysis by thin-layer chromatography (TLC), acid hydrolysis and methylation as described by Schlenk and Gellerman.22
Biochemical analyses of TG and total cholesterol were performed as previously described.23 Briefly, triglycerides were measured using a glycerol blanked enzymatic method (Trig/GB, Roche Diagnostics Corporation, Indianapolis, IN) and cholesterol was measured using a cholesterol esterase, cholesterol oxidase reaction (Chol R1, Roche Diagnostics Corporation) on the Roche/Hitachi 911 Automatic Analyzer (Roche Diagnostics Corporation).
Statistics
All data were analyzed using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). First, nonparametric tests (one-sample Kolmogorov-Smirnov test) were used to determine whether parameters were consistent with normal distribution and Pearson correlation coefficients were calculated to assess the correlations. Statistical significance was set at P, 0.05 for all tests.
General data
The clinical characteristics of the male study population are represented in Table 1, by age group, BMI (kg/m2), semen volume (ml), total sperm count (106), Sperm concentration (106), motility (%) and morphology (%). There were no significant differences between male study population and controls, except for total sperm count (p<0.001), sperm concentration (p<0.001), motility (p<0.01) and morphology (p<0.01). In fact, the percentage of sperm with normal count, motility and morphology was higher for the control males than for the male study population.
|
Male patients (n=20) |
Sperm donors (n=39) |
P value |
Age (Years) |
35.5±10 |
32.0±12 |
NS |
BMI (kg/m2) |
22.1±4 |
23.1±6 |
NS |
Semen volume (ml) |
3.2±1 |
2.85±1.5 |
NS |
Total sperm count (x106) |
34±3,5 |
156±19 |
0.001 |
Sperm concentration (x106) |
10,2±4,1 |
87±15 |
0.001 |
Motility (%) |
24±6 |
58±10 |
0.01 |
Normal morphology (%) |
3±1 |
35±10 |
0.01 |
Table 1 Characteristics of male patients (n=20) and fertile volunteers (n=39) in terms of age, BMI (kg/m2), semen volume (ml), total sperm count (106), sperm concentration (106), motility (%) and morphology (%). There were no significant differences between male study population and controls, except for total sperm count (p<0.001), sperm concentration (p<0.001), motility (p<0.01) and morphology (p<0.01)
Values are mean ± SD;
BMI, body mass index;
The clinical characteristics of the female study population are represented in Table 2, by age group and response levels in terms of BMI (kg/m2) anti-Müllerian hormone (AMH), amount of basal FSH, amount of administered FSH, the oestradiol value on the day of hCG/GnRH injection and number of oocytes retrieved. No statistical difference was found in the amount of total gonadotrophin used for ovarian stimulation in poor responders compared with the non-poor responders group. Serum oestradiol concentrations on the day of the HCG injection were lower in poor responders (P=0.01) (Table 2). As the response levels were defined according to oocyte numbers, the number of retrieved oocytes was significantly different in between the poor and non-poor responders (P=0.001).
|
Female patients (n=14) |
Oocyte donors (n=40) |
P-value |
Age (Years) |
32.4±2.1 |
29.4±4.1 |
NS |
BMI (kg/m2) |
22.5±2.1 |
21.4±1.3 |
NS |
AMH (ng/ml) |
3.66±1.2 |
4.74±1.3 |
NS |
Basal FSH (IU/l) |
5.2±1.2 |
5.7±3.2 |
NS |
Total FSH administered (IU) |
1877±158 |
1962±146 |
NS |
E2 value of hCG/GnRH day (pg/ml) |
1547±198a |
2473±361 |
0.01 |
Total oocytes retrieved |
3.2±1.3b |
11.5±3.8 |
0.001 |
Table 2 Patients characteristics in the low responders (<5 oocytes)(n=14), and control groups (oocyte donors)(n=40) in terms of BMI (kg/m2) anti-Müllerian hormone (AMH), amount of basal FSH, amount of administered FSH, the oestradiol value on the day of hCG/GnRH injection and number of oocytes retrieved. No statistical difference was found in the amount of total gonadotrophin used for ovarian stimulation in poor responders compared with the non-poor responders group. Serum oestradiol concentrations on the day of the HCG injection were lower in poor responders (P = 0.01). As the response levels were defined according to oocyte numbers, the number of retrieved oocytes was significantly different in between the poor and non-poor responders (P = 0.001)
Values are mean ± SD
BMI, body mass index;
AMH, anti-mullerian hormone;
The value of Estradiola and number of retrieved oocytesb are significantly different among the poor and non-poor responder patients.
Relationship between lipids levels in serum and seminal plasma of male patients and sperm donors
Subjects with low sperm motility and sperm concentration had higher median serum lipid concentrations than those without sperm alterations. In the group of patients, the levels of TG and NEFA in seminal fluids and TG in plasma were significantly elevated as compared to the control group (TG: 91.1±7.3mg/dl in serum donors and 164.3±18.7mg/dl in serum patients, p<0.01; 93.6±8.94mg/dl in seminal plasma donors and 200.3±53.36mg/dl in seminal plasma patients, p<0.01; NEFA: 7.8±0.078mg/dl in seminal plasma donors and 11.4±0.097mg/dl in seminal plasma patients) (Figures 1&2).
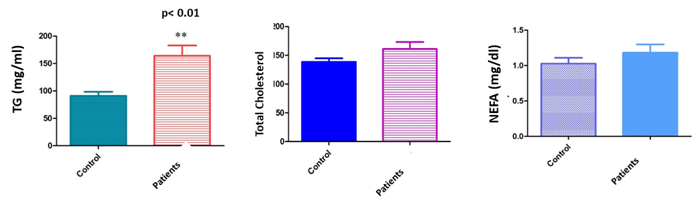
Figure 1 Level of lipids profile (TG, cholesterol and NEFA) in serum of male patients compared with controls (sperm donors). Male patients had higher median serum lipid concentrations than those without sperm alterations. In the group of patients, the levels of TG in plasma were significantly elevated as compared to the control group (TG: 91. 1±7.3mg/dl in serum donors and 164.3±18.7mg/dl in serum patients, p<0.01; No statistically significant differences were observed in between total cholesterol levels in serum from patients and sperm donors. Likewise, no correlation was either found in between NEFA levels in serum and sperm quality.
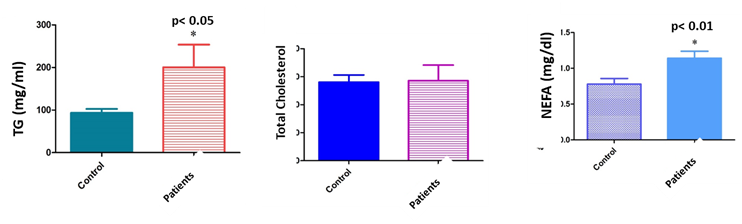
Figure 2 Level of lipids profile (TG, cholesterol and NEFA) in seminal plasma of male patients compared with controls (sperm donors). In the group of patients, the levels of TG and NEFA in seminal fluids were significantly elevated as compared to the control group (93.6±8.94mg/dl in seminal plasma donors and 200.3±53.36mg/dl in seminal plasma patients, p<0.01; NEFA: 7.8±0.078mg/dl in seminal plasma donors and 11.4±0.097mg/dl in seminal plasma patients). No statistically significant differences were observed in between total cholesterol levels in seminal plasma from patients and sperm donors.
No statistically significant differences were observed in between total cholesterol levels in serum or seminal plasma from patients and sperm donors. Likewise, no correlation was either found in between NEFA levels in serum and sper.
Relationship between lipid levels in serum and follicular fluid of female patients and oocyte donors
TG in follicular fluid and NEFA concentrations in serum and follicular fluid were significantly higher in patients with low response than in the control group. (TG: 100.8±8.25mg/dl in follicular fluid donors and 137.1±10.47mg/dl in follicular fluid patients, p<0.01; NEFA: 3.5±0.035 mg/dl in FF donors and 6±0.1mg/dl in FF patients; 3.1±0.013mg/dl in serum donors and 4.7±0.08 mg/dl in serum patients) (Figures 3&4).
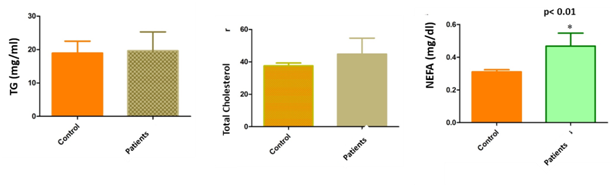
Figure 3 Level of lipids profile (TG, cholesterol and NEFA) in serum of female patients compared with controls (sperm donors). NEFA concentrations in serum were significantly higher in patients with low response than in the control group. (3.1±0.013mg/dl in serum donors and 4.7±0.08mg/dl in serum patients). No statistically significant differences were observed in between TG and total cholesterol levels in serum from young women with LR and oocyte.
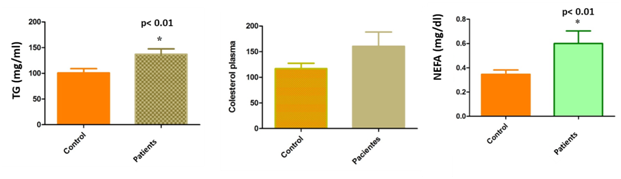
Figure 4 Level of lipids profile (TG, cholesterol and NEFA) in follicular fluid of female patients compared with controls (sperm donors). TG in follicular fluid and NEFA concentrations in follicular fluid were significantly higher in patients with low response than in the control group. (TG: 100.8±8.25mg/dl in follicular fluid donors and 137.1±10.47mg/dl in follicular fluid patients, p<0.01; NEFA: 3.5±0.035mg/dl in FF donors and 6±0.1mg/dl in FF patient). No statistically significant differences were observed in between total cholesterol levels in FF from young women with LR and oocyte.
No statistically significant differences were observed in between total cholesterol levels in serum neither in FF from young women with LR and oocyte.
Several studies showed significant correlation in between lipid composition in sperm membrane and seminal plasma.24 as well as increased phospholipid levels in seminal plasma in oligospermic and azoospermic patients.25 Studies on lipid composition of human testis in patients with bilateral varicocele as cause of infertility showed abundant lipids in the testis tissue. The total amount of lipids was 1.90percent of the total wet weight of the human testis tissue. Testicular cholesterol, glycerides and phospholipids were 26.50 percent, 28.50 percent and 45percent, respectively, of the total percentage of lipids.26 Also abundant cholesterol and phospholipids were found in sperm membranes,27 especially polyunsaturated fatty acids (PUFA) closely related to sperm function.28‒31 During spermatogenesis, sperm maturation, capacitation and acrosome reaction, lipid composition in sperm membranes change significantly.32‒34 These changes were possibly based on the transfer of cholesterol and phospholipids in between sperm and seminal plasma.35‒36 However, no clear correlation was found in between lipid levels in serum and seminal plasma with semen parameters.
In the present study, we analyzed the correlation in between lipid levels in serum and seminal plasma and semen parameters in 20 infertile men compared with fertile sperm donors. We have found a correlation in between levels of triglycerides both in serum and sperm, and sperm quality. A relationship was also found in between non esterified fatty acids in seminal plasma and sperm quality, suggesting that male infertility could be influenced by lipid metabolism. However, surprisingly, serum and seminal plasma levels of total cholesterol were unrelated to semen parameters. This observation was similar to the results reported by Hagiuda and colleagues.37 The fact that higher concentrations of NEFA were found in seminal plasma but not in blood suggests that Lipid levels may not be originiated from blood/the origin of these lipids might be different to blood. This raises the question about how lipids are originated and regulated in seminal plasma. In fact, it may be possibly originated from epithelial cells in the male reproductive tract.
Further, we analyzed the correlation of lipids levels in serum and follicular fluid with female infertility (number of oocyte and response to ovarian stimulation). The results showed that the level of NEFA in serum as well as in follicular fluid and triglycerides in follicular fluid were slightly higher in patients compared with oocyte donors.
Within a growing follicle in the ovary, the oocyte is surrounded by concentric layers of cells and tissue. These layers are, from the periphery inwards:
Our study shows that the level of triglycerides is higher in the follicular fluid of patients with low response than in fertile donors, which can be translated as a malfunction of the follicle in these patients. However, the possibility that granulosa cells secrete triglycerides has not been investigated previously. Our results are in agreement with those published by Robker et al.,40 which showed a relationship in between obesity and increased triglycerides in the follicular fluid. It is likely that additional factors also associate with high lipid content in follicle fluid and contribute to the dramatic effects on oocyte maturation. For instance, inflammatory mediators and oxidative stress are elevated in the follicular fluid of obese women and, as we demonstrated, in low responders patients.
Our study reveals that lipid levels in follicular fluid vary remarkably between women. Also that differing levels of these lipids, and presumably many additional cofactors in follicular fluid, can exert dramatically different effects, as previously showed by Yang et al.41
Lipids levels in the patient group (both, men and women) are superior to the control group probably due to a compensatory mechanism caused by a possible increase in oxidative stress.
In conclusion, altered sperm parameters and low ovarian reserve are associated with elevated triglycerides and fatty acids in seminal plasma and ovarian follicular fluid. Gamete maturation within this lipid-rich environment is detrimental to spermatozoa and oocytes.
None.
The authors declare that there is no conflict of interest.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.