eISSN: 2473-0815


Background: Diabetes mellitus type 1 (T1DM) is the most common endocrine metabolic disorder in children and adolescents. Age, gender, race, genotype, seasonality and viral infection are the main T1DM risk factors that have been identified.
Aim of the study: was to describe the clinical characteristics and laboratory findings and the different risk factors in infants who developed diabetes mellitus during the first two years of their life.
Methods: A case control study was conducted in pediatric outpatient endocrine clinic, Zagazig University Hospitals. It included 34 diabetic infants and 34 healthy infants in the 1st 2 years of life. For all subjects, we performed random blood sugar, glycosylated hemoglobin (HbA1C) and anti-islet cell antibody by indirect immunofluoresent assay.
Results: More than two thirds of the studied type 1 diabetic infants were detected at 12-24 months of age. There was no statistical significant association between predisposing risk factors of type 1 DM and the age of onset except for high birth weight (HBW) and positive family history of DM (27.3% and 90.9% respectively) at <12 months of age (p <0.05). Polyuria, polydipsia, weight loss, and diabetic ketoacidosis (DKA) were the most frequent presentations among the studied type 1 diabetic patients (93.3%, 73.3%, & 53.3% respectively). There was statistical significant differences in the means of RBG & HbA1C among cases and controls (p <0.05).Fasting serum C-peptide level was significantly lower among type 1 diabetic infants at age 12 months or more (p <0.05). Islet cell antibodies were negative in both patients and controls by indirect immunoflourescene assay.
Conclusion: Avoidance of predisposing factors in infantile DM may help to delay the onset of disease but does not prevent it especially if genetically determined.
Keywords: infantile; diabetes mellitus; risk factors; ethical consideration; presented; summarized; tabulated; analyzed; heterogeneous; patients; cellular basis; cell maturation; glucose-intolerance; autoimmunity; risk factors
ICA, islet cell antibody; GAD, glutamic acid decarboxylase; IFA, iindirectimmuno fluorescent assay
Diabetes mellitus is a metabolic disease characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. The chronic hyperglycemia of diabetes is associated with long term damage, dysfunction and failure of various organs, especially the eye, kidney, nerves and blood vessels.1 The onset of type 1 diabetes before the first year of age is a rare condition and is probably due to an interaction between genetic and environmental factors, which together may explain such an early event.2
Environmental triggers in infancy and early childhood may accelerate the onset of diabetes. For example, enteroviral infection -documented by polymerase chain reaction was detected in twins developing type 1 diabetes in infancy, before detection of islet cell antibodies.3
To date, 14 different viruses -including picorna viruses, rotaviruses, herpes viruses, mumps, rubella and retroviruses, have been reported to be associated with the development of T1DM.4
Beta cell autoimmunity is increased in children who are not breast fed for a short time. Early introduction of cow' milk based formula increases the risk for developing type 1 diabetes up to 5 years of age in the general population.5
Many autoantibodies against 13 cells have been identified. The most important ones are: islet cell antibody (ICA), Anti- insulin anti Glutamic acid decarboxylase (GAD) and the anti-body against the tyrosine phosphatase (PTP ) -1 protein known as ICA - 512 (IA-2).6
Permanent neonatal diabetes mellitus is less common than transient form. By definition, diabetes develops in the neonatal period and does not go into remission.7
The current study aimed to describe the clinical characteristics and laboratory findings and the different risk factors in patients who developed diabetes mellitus during the first two years of their life.
A case control study was conducted at Zagazig city over the period of 12 months from June 2014 to May 2015.
Target population
Cases: type 1 diabetic infants (≤2years old) attend the pediatric outpatient endocrine clinic in Zagazig University hospital and Aswan University Hospitals.
Control group: Healthy infants (≤2years old) attending the Maternal & child health care centers (El-Nahal MCH center) for vaccination.
Inclusion criteria for cases: diagnosed Type 1 diabetic infants, with age ≤2 years and attending pediatric outpatient endocrine clinic in Zagazig university hospital and Aswan University Hospitals.
Inclusion criteria for control group: Healthy infants with age ≤2 years & no comorbid chronic diseases.
Sample size
Assuming that expected family history of DM among cases & controls were (27.1%, & 1% respectively)8 and Odds ratio=36.8, at 95% confidence interval & 80% power of the study. So the calculated sample size equal 68 infants which was divided into 34 cases & 34 controls.
Study tools
Ethical consideration: This study was approved by the ethical committee of Zagazig University, Egypt and written informed consent from parents was provided in accordance with the Declaration of Helsinki.
Data management: The collected data were presented, summarized, tabulated & analyzed by using computerized software statistical packages (EPI-info Version 6.04 & SPSS version 19). P<0.05 was considered to be statistically significant. Chi square & Fisher exact tests were used to compare proportions. Two samples t- test was used to compare means between the two studied groups.
Table 1 showed that the mean age & SD of type 1 diabetic patients was 18.4 + 10.8 with age range (2-24 months), versus to that 16.5 + 8.2 & age range (4-23 months) among the control group. Also there was no statistical significant differences between cases & controls regarding weight and height percentiles (p>0.05).
Variable |
Cases Mean + SD |
Controls |
P Value |
Age (Months) |
18.4 + 10.8 |
16.5 + 8.2 |
>0.05 |
Gender |
|||
Males |
14 |
18 |
>0.05 |
Females |
20 |
16 |
|
Weight (Percentile) |
41 + 25.8 |
45.7 +20 |
>0.05 |
Height (Percentile) |
50.7 + 24.7 |
53.7 + 22.9 |
>0.05 |
Table 1 Comparing mean age, weight, & height among the studied cases and controls
p<0.05 significant.
More than two thirds of the studied type 1 diabetic infants were detected at 12-24 months of age as demonstrated in Figure 1.
Polyuria, polydipsia, weight loss, and DKA were the most frequent presentations among the studied type 1 diabetic patients (93.3%, 73.3%, & 53.3% respectively) as illustrated in Figure 2.
Figure 3 revealed that; introduction of cow’s milk before 1 year and positive family history of DM were the most prominent & statistically significant predisposing risk factors for type 1 diabetes among the studied groups (p<0.05).
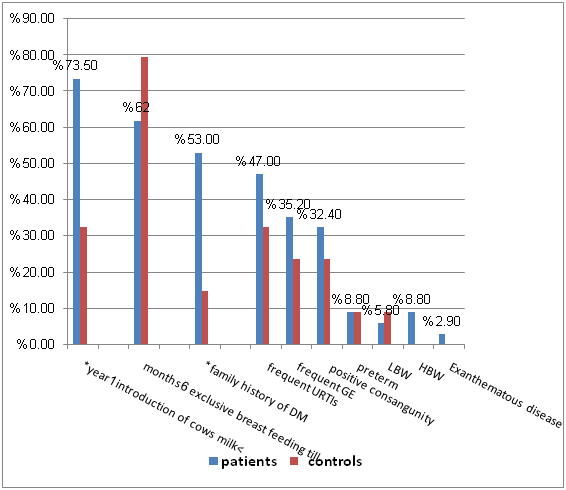
There was no statistical significant association between predisposing risk factors of type 1 DM and the age of onset except for HBW and positive family history of DM (27.3% & 90.9% respectively) at <12 months of age (p <0.05) as shown in Table 2.
Predisposing Factor |
Age of Onset |
P Value |
|
<12 Months |
≥12 Months |
||
n (11) % |
n (23) % |
||
Birth Weight: |
|||
LBW |
1 9.1 |
1 4.3 |
>0.05 |
HBW |
3 27.3 |
0 0.0 |
<0.05* |
Gestational age: |
|||
Preterm |
2 18.2 |
1 4.3 |
>0.05 |
+ve family history of DM |
10 90.9 |
8 34.8 |
<0.05* |
+ve consanguinity |
6 54.5 |
5 21.7 |
>0.05 |
Exclusive breast feeding till 6 months |
9 81.8 |
12 52.2 |
>0.05 |
History of introduction of cow’s milk <1 year |
10 90.9 |
15 65.2 |
>0.05 |
Frequent URTIs |
6 54.5 |
10 43.4 |
>0.05 |
Frequent gastroenteritis |
5 45.5 |
7 30.4 |
>0.05 |
History of exanthematous diseases |
0 0.0 |
1 4.3 |
>0.05 |
Table 2 Association between the age of onset of type 1 DM and predisposing risk factors among the studied patients
p<0.05 significant
As regards to mean & SD of random blood glucose and glycosylated hemoglobin among the studied cases & controls, There was statistical significant differences in the means of RBG & HbA1C among cases and controls (p <0.05) (table 3). Fasting serum C-peptide level at the beginning of diagnosis was significantly lower among type 1 diabetic infants at age 12 months or more (p <0.05) as in Table 4. Figure 4 illustrated that 88.2% of the studied patients was receiving insulin dose < 1 u/kg/d.
Variable |
Cases |
Controls |
P Value |
||
Mean + SD |
Range |
Mean + SD |
Range |
||
RBG (mg⁄dl) |
327 + 91.8 |
187-503 |
97.4 +7.0 |
85-110 |
<0.05* |
HbA1c |
7.6+ 1.1 |
5.7-8.6 |
5.2 +0.6 |
4-6.3 |
<0.05* |
Table 3 Mean & SD of random blood glucose and glycosylated hemoglobin among the studied cases & controls
p<0.05 significant*
Variable |
Age of Onset of Type 1 DM |
P Value |
|||
<12 Months n (11) |
≥12 Months n (23) |
||||
Mean + SD |
Range |
Mean + SD |
Range |
||
Fasting C-peptide |
0.32+ 0.04 |
0.3-0.4 |
0.23 +0.08 |
0.1-0.4 |
<0.05* |
Table 4 Relation between fasting serum C-peptide at the beginning of diagnosis and the age of onset
p<0.05 significant*
Diabetes mellitus is a rare disorder during the first 2 years of life, amounting to about 3-5% of all cases diagnosed before the fifteenth birthday. However, in spite of low numerical values, this is an important diagnosis, since we are dealing with a vulnerable age group with major and special problems related to diagnosis, treatment and psychological follow up.10
Our study showed that there was female predominance in infantile onset diabetes 20 female patients (58.9%) versus 14 male patients (41.1%) that comes in agreement with the results of Kumar et al.,11 who reported that there is a marked female predominance in infantile onset diabetes.
In our study +ve family history of diabetes was significantly different between patients and controls (53% patients versus 15% controls) (p value<0.05). That came in agreement with the results of Hathout et al.,3 who reported-that neonates, infants and toddlers with type 1 diabetes are more likely to have a father with type 1 diabetes than diabetic children diagnosed at a later age.
Sipetic et al.,12 and Marshall et al.,13 reported that +ve family history of type 1 diabetes has-consistently been reported to raise type 1 diabetes risk among relatives. These results were in contrast with the study of Edna- et al.,14 that described the abrupt onset of type 1 diabetes and the absence of family history.
Positive consanguinity was more frequent in patients than in controls but not reaching significant level (33.3% patients versus 20% controls) & no significant correlation between consanguinity and earlier onset of the disease.
In our work we found that 47% of patients had frequent upper respiratory tract infections, 35.2% of patients had frequent gastroenteritis and 2.9% had exanthematous disease before onset of DM. Infections were more frequent in patients than in controls but not reaching significant level.
Infant diet has been hypothesized to be involved in the initiation of the type 1 diabetes autoimmune process by impairing the maturation of the gut associated immune system &/ or by providing antigens cross-reactive to islet cell antigens (molecular mimicry).15 However, infant diet may affect diabetes risk as well through pathways according to the recently raised [accelerator hypothesis] or the [over load hypothesis] (bottle -fed children are more likely to develop childhood adiposity).16
In the present study, we found that introduction of cow milk < one year was significantly different between patients and controls (73.5% in patients & 33.3% in controls) P value (<0.05). That came in agreement with results of Holmberg et al.,5 who reported that early introduction of cow milk based formula increases the risk for developing type 1 diabetes up to 5 years of age in the general population. Also, Karjalainen et al.,17 reported that, in susceptible families early exposure to cow' s milk proteins can increase the risk of the infant or child developing type 1 diabetes mellitus. However, the work of Wasmuth & Kolb18 did not support this conclusion by reporting that several epidemiological studies and more importantly, the first prospective trials did not show an association between early exposure to cow's milk and type 1 diabetes mellitus.
In the current study we found that no significant difference between patients and controls as regards birth weight. LBW (5.8% patients versus 8.7% controls) & HBW (8.8% patients versus 0% controls) and also we found no significant correlation between birth weight and the earlier onset of diabetes that came in agreement with Mckinney et al.,19 who reported that no association between birth weight specially (LBW) and diabetes mellitus but no association between LBW and earlier onset of diabetes. However, the study of Stene et al.,20 observed that LBW to be associated with an increased risk of diabetes Mellitus & Kibirige et al.,21 reported that LBW is associated with earlier onset age of diabetes.
The highest percent of our patients were presented by polyuria/polydepsia weight loss, and DKA (93.3% , 73.3%, and 53.3% respectively).
This came in agreement with the work of Hathout et al.,3 showed a higher frequency of diabetic ketoacidosis in the early onset group at the time of presentation. Joseph et al.,22 reported that even in developed countries, 15-70% of all newly diagnosed infants and children with diabetes present with DKA.
In our work, we found that there was negative significant correlation between level of fasting C - peptide and age of onset of the disease (p value < 0.05). The earlier age of onset of the disease the higher level of fasting C-peptide.
We found that all patients and controls were negative for islet cell antibodies. This was in accordance with Mustonen et al.,23 who reported that there was strong association between HLA antigens (A9 & Bw16 ) and an absence of ICA early ( within two years ) in the course of the disease. They also reported that the role of ICAs in the pathogenesis of type 1 DM is not known. Patients without ICA may represent a group with a different etiology of type 1 DM, they could be more susceptible to some environmental factor or factors than others. On the other hand the ICA negativity may just reflect differences in the patterns of immunological responsiveness after islet cell damage between patients groups, without any association with the pathogenesis of the disease.
Metz et al.,24 also reported: in T1DM tests are negative for anti-islet antibodies and for HLA class II haplotypes conferring susceptibility to type 1 diabetes. A defect in cell maturation has been suggested. Interestingly exocrine pancreatic insufficiency is present in only a few patients. However, the cellular basis of TIDM remains unknown. Most patients recover within a year, but a few have persistent glucose-intolerance and/or recurrence of diabetes in late childhood or adulthood.
Moreover, Salma25 also reported that Type 1 b (idiopathic type 1) refers to rare forms of the disease with no known cause. There is no evidence of autoimmunity; it represents about 10% of cases of type 1 in Europe. It may be etiologically heterogeneous, including insulin secretory defects caused by extensive pancreatic islet B-cell destruction or dysfunction.
In conclusion, we focused on an important age group of diabetic patients with early onset of the disease and the importance of avoiding exposure to the different risk factors which may precipitate early onset of diabetes. This may help to delay such an early onset but does not-prevent it especially if it is genetically determined. So our recommendations are Improving awareness and knowledge of parents or care givers about risk factors of type 1 DM, its main presentations and the importance of regular follow up to assess the effectiveness of treatment and to prevent development of complications.
None.
The author declares there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.