eISSN: 2473-0815


Background: Salacia Chinensis (SC) is herbal medicine in Ayurvedic medicine. The extract of Salacia chinensis showed preventive effects on various metabolic health disorders.
Objective: The aim of this study was to elucidate the therapeutic efficacy of the extract of Salacia chinensis extract (SCE) and modulation of carbohydrate and lipid metabolism in in vitro and in vivo models.
Methods: In vitro studies were conducted to study the effect of Salacia chinensis extract (SCE, OmniLeanTM) on alpha-glucosidase, pancreatic lipase and HMG-CoA reductase. The activity of α-glucosidase was assessed using mammalian α-glucosidase extracted from rat intestinal acetone powder (Sigma) and p-nitrophenyl L-D glucopyranoside (PNPG) as an artificial substrate. Pancreatic lipase activity was assessed using pancreatic porcine lipase and 4-methylumbelliferyl oleate (MU Oleate) as substrate. Lipase activity was measured using a fluorescence kinetic assay. HMG-CoA reductase was the rate-limiting step in cholesterol synthesis. The activity of HMG-CoA reductase was assessed using HMG-CoA reductase assay kit (Sigma CS1090). Nutrigenomics study was conducted to study the effect of SCE on fatty acids, PPAR gamma and C/EBP α. In an in vivo model, fifty-six (56) male C57BL/6 mice were purchased from Charles River Labs, at the age of 5 weeks. The mice were housed individually in mouse cages in a temperature-controlled room with a 12-hour light and dark cycle and free access to regular rodent chow and water. After a week of adaptation, the mice were divided into 3 groups with 8 mice in each group. All mice were fed a high-fat diet (60% energy from fat) to induce obesity. One of the group was administered with low (100mg/kg/d) and high (500mg/kg/d) doses of SCE suspending in 0.5% carboxymethyl cellulose, respectively for 7.5 weeks (52 days). One group was used as the obese control (N=8) and gavage with the vehicle control. Body weights were obtained daily and food intake was recorded thrice every week. Fecal samples were collected during week 6 and 7. Serum lipid analysis, glycerol, free fatty acids, hormonal assays (ghrelin, adiponectin, leptin, insulin, glucose and GLP), fecal crude lipids and histopathology changes were assessed.
Results: Salacia chinensis extract (SCE) showed a significant inhibitory effect on α-glucosidase. SCE demonstrated a significant inhibitory effect against pancreatic lipase, compared to orlistat. SCE inhibited HMG-C0A only at higher concentration (1mg/ml). In nutrigenomics model, SCE upregulated C/EBP alpha and down regulated ACC, PPARG and SCD-1. SCE at the dose of 500mg/kg/d significantly slowed body weight gain, reduced food intake, increased adiponectin, decreased fat pads mass and increased fecal fat excretion in animal model. SCE significantly decreased significantly serum glycerol levels (by 69%) and free fatty acids levels (by 77%) compared to control group. Treatment with SCE decreased by 20% leptin levels and by 21% insulin levels and increases by 16% adiponectin but did not affect glucose or GLP-1 levels.
Conclusion: In vitro results suggest the effects of SCE in modulating gastrointestinal carbohydrate and lipid digestion and absorption, may be advocated as a candidate for obesity-diabetes prevention and phytotherapy. Animal study (in vivo) results suggest the inhibition of fat absorption slows weight gain and reducing effects of metabolic risk factors with SCE. More experiments need to be performed to optimize the optimal concentration that has an effect in vivo on body weight or food intake.
Keywords: salacia Chinensis, carbohydrates, lipids, metabolism, body weight, obesity, diabetes, α-glucosidase, hyperlipidemia
SCE, salacia chinensis extract; FBP, fructose-1,6-bisphosphatase; FBS, fasting Blood sugar; DMEM, dulbecco modified eagle’s medium; FBS, fetal bovine serum; HSL, hormone sensitive lipase; BMI, body mass index; FE, food efficiency; C/EBP alpha, CCAAT/enhancer BINDING protein alpha; ACC alpha, Acetyl-CoA carboxylase alpha; PPARƔ, peroxisome proliferator-activated receptors gamma; SCD-1, stearoyl-COA desaturase-1; phospho-HSL, phosphorylated hormone sensitive lipase; L: low; H, high; GLP-1, glucagon-like peptide-1
Current incidence and prevalence of obesity and overweight rates are more than a third of adults. Overweight and obesity related to co morbidity was observed in children and teenagers.1 It was estimated and projected that one in three adults could have diabetes in a house hold by 2050.2 Diabetes is a chronic, progressive disease characterized by elevated levels of blood glucose and lead to micro and macro vascular complications.3 Obesity and diabetes may lead to comorbid conditions such as hypertension, cardiovascular disease, cancer and other chronic inflammatory conditions. Novel nutritional interventions and some of the functional ingredients are in various stages of development to show significant effects on weight management and anti-diabetic properties. S chinensis is a common species abundantly found and a good number of specimens from Karnataka, Goa and Maharashtra are deposited. This species is called ‘Eka Nayaka’ in Kannada. This species is also commonly known as Saptrangi, Dimal, Modhupal, Ingli, Cherukuranti, Nisul-bondi. This species is a small erect or straggling tree or large, woody, climbing shrub found throughout India including the Andaman and Nicobar Islands. The plant is harvested from the wild for local use as a food and therapeutic purposes. The S. chinensis plant is a woody climber found in Sri Lanka and India. Salacia Chinensis (SC) is considered orally effective in the treatment of diabetes. Salacinol and kotalanol were reported to inhibit α-glucosidase activity, while Mangiferin was reported to decrease the expression of fructose-1,6-bisphosphatase (FBP), a key enzyme involved in gluconeogenesis in the liver.4
Recent preclinical and clinical studies have demonstrated that Salacia roots modulate multiple targets: peroxisome proliferator-activated receptor-alpha-mediated lipogenic gene transcription, angiotensin II/angiotensin II type 1 receptor, alpha-glucosidase, aldose reductase and pancreatic lipase. These multi-target actions may mainly contribute to Salacia root-induced improvement of type 2 diabetes and obesity-associated hyperglycemia, dyslipidemia and related cardiovascular complications seen in humans and rodents.5-9 Salacia appears to have a fairly unique polyphenolic profile. Salacia tends to contain Salacinol, Salaretin, Mangiferin, Kotalanol, Triterpenesand, 13 MRT and Ponkoranol.10,11 Alpha-glucosidase inhibitors decrease the absorption of carbohydrates from the intestine, resulting in a slower and lower rise in blood glucose throughout the day, especially right after meals. They are important constituents of the traditionally used anti-diabetic medicines, and are also therapeutic for other carbohydrate-metabolic disorders.
Prediabetes and mild to moderate hyperlipidemia received either Salacia extracts (500mg/day) or placebo along with therapeutic lifestyle changes for a period of 6 weeks. Improvements in lipid profiles and glycemic levels were observed in Salacia extract-treated groups when compared to placebo at week 6. A statistical significant reduction was observed in low-density lipoprotein cholesterol and fasting blood sugar (FBS) levels at week 3 and 6 when treated with root bark extract. The leaves extract-treated group showed statistically significant reduction in FBS levels at week 6 only.12 The mRNA expressions of lipogenesis factor (peroxisome proliferator-activated receptor γ, lipoprotein lipase, CD36, and fatty acid binding protein 4) were down-regulated, while the expressions of lipolysis factor (adipose tissue triacylglycerol lipase and HSL) and adiponectin were up-regulated. S. reticulata enhanced the expression of total AMP-activated protein kinase α (AMPKα) and phosphorylated AMPKα in mature adipocytes.13 An aqueous extract of Salacia Reticulata, in rats fed 10-75mg/kg of it, appears to be able to reduce the spike in blood glucose from sucrose or maltose in a relatively dose-dependent manner.14 In a randomized, double blind, parallel group, placebo controlled trial, Salcital (500mg/d) for a period of 6 weeks along with therapeutic lifestyle changes treated group showed significant reduction in LDL-C and fasting blood sugar levels when reduction in LDL-C and fasting blood sugar levels when compared to placebo in patients with prediabetes and mild to moderate hyperlipidemia.8 A similar product of Salacia species (Salacia oblonga) noted that, in 66 overweight type II diabetic persons given either 240mg or 480mg of a Salacia Oblonga extract (ethanol and water extract) prior to a sugary meal (maltodextrin, sucrose, corn syrup; 110g total), that the extract was able to reduce the peak postprandial glucose reading by 19% and 27% (240mg and 480mg; respectively) and reduced the 3-hour AUC by 14% and 22%; following this reduction in glucose was a reduction in serum insulin, reducing the peak by 9 and 12% and the 3 hour AUC by 14% and 19% in type 2 DM.15 Yoshino et al.,16 reported an oral pre-administration of extracts of Salacia leaves and stems at doses of 100, 200 and 400mg/kg body weight prevented the elevations of lipid peroxides and injury markers in a dose-dependent manner. Decrease of antioxidant activity in plasma of mice treated with Fe-NTA was also prevented by administration of these extracts.
The aim of this study was to elucidate the therapeutic efficacy of the extract of Salacia chinensis extract (SCE) and modulation of carbohydrate and lipid metabolism in in vitro and in vivo models.
Enzyme assays such as alpha-glucosidase, pancreatic lipase HMG-CoA reductase, lipolysis and adipogenesis assays were conducted. In vitro models and an in vivo study was conducted to understand Salacia chinensis extract (SCE) mechanism of action and its potential role to induce weight loss or prevent weight gain.
Investigational product
Salacia chinensis extract (SCE, OmniLean) provided by OmniActive Health Technologies Ltd., India.
Enzyme assays
Alpha-glucosidase assay: The objective of this assay is to assess the prevention of carbohydrates absorption. The activity of α-glucosidase is assessed using mammalian α-glucosidase extracted from rat intestinal acetone powder (Sigma) and p-nitrophenyl L-D glucopyranoside (PNPG) as an artificial substrate. Acarbose is used as a positive control. Αlpha-glucosidase activity is determined using a colorimetric kinetic assay by monitoring the release of PNPG at the absorbance 400nm using a microplate reader Synergy H1.
Pancreatic lipase assay: The objective of this assay is to assess the extract ability to prevent fat absorption. Pancreatic lipase activity is assessed using pancreatic porcine lipase and 4-methylumbelliferyl oleate (MU Oleate) as substrate. Orlistat, is used as a positive control. Lipase activity is measured using a fluorescence kinetic assay.
HMG-CoA reductase assay: HMG-CoA reductase is the rate-limiting step in cholesterol synthesis. The activity of HMG-CoA reductase was assessed using HMG-CoA reductase assay kit (Sigma CS1090). It measures the oxidation of NADPH by the catalytic subunit of HMG reductase in the presence of the substrate: HMG-CoA at the absorbance 340nm.
In Vitro studies-3T3L1 preadipocytes
Lipolysis: 3T3L1 preadipocytes were cultured for a week in Dulbecco modified Eagle’s medium (DMEM) supplemented with 10% new calf serum, 25mM glucose, 2mM glutamine, 100U/ml penicillin, 100ug/ml streptomycin at 37°C in 5% CO2 atmosphere until 100% confluence. Two days after confluence, the differentiation of 3T3L1 into adipocytes was initiated using (DMEM) supplemented with 10% fetal bovine serum (FBS), 25mM glucose, 2mM glutamine, 100U/ml penicillin, 100ug/ml streptomycin and differentiation inducers (0.5mM isobutyl-methylxanthine (IBMX), 10µg/ml insulin and 1µM dexamethasone) for 48h (day 0-day 2). The cells were then cultured for another 48h with the same medium containing only 10µg/ml insulin (day 2 to day 4), then with only the medium (day 4 to day 6). SCE was added to the medium at the concentration 100µg/ml for 24h and assessed for lipolysis. The effect of the SCE was compared to a positive control isoproterenol (10uM). The glycerol released after 24h in the cell media was assessed using glycerol reagent (sigma) and expressed relative to the cellular protein content. The protein content was assessed using BCA Pierce kit. If the extract displays a ratio glycerol/protein higher than 1.40, it is considered as an extract that enhance lipolysis. Proteins were collected from cell lysates of 3T3L1 adipocytes treated for 24h with SCE or isoproterenol. Protein samples were blotted and probed for total hormone sensitive lipase (HSL), and phosphorylated hormone sensitive lipase at Serine 563; the ratio of phosphor-HSL to total HSL was compared between treated and non-treated samples.
Adipogenesis: 3T3L1 preadipocytes were treated with control or the extract at 3 concentrations (25, 50 and 100ug) from days 0 to 6 of differentiation, using the standard differentiation protocol (insulin, dexamethasone, 3-isobutyl-1-methylxanthine). The differentiation of adipocytes is accompanied by the accumulation of intracellular droplets of triglycerides. The effect of the extracts on adipogenesis was evaluated by measuring the intracellular lipid accumulation using AdipoRed assay reagent.
Nutrigenomics
3T3 T3-L1 murine adipocytes are a common model system used to understand basic cellular mechanisms associated with diabetes, obesity and related disorders. 3T3-L1 preadipocytes and media for proliferation, differentiation, and maintenance were purchased. Cells were cultured until they were confluent and differentiated to mature adipocytes (approximately 18 days). These adipocytes were treated overnight with three compounds [OmniLean, SCE from OmniActive Health Technologies Ltd. and two commercial products) as outlined in the table. Subsequently, RNA was isolated and cDNA prepared using standard protocols and real-time PCR performed on a select list of genes using standard protocols.3,4
The primers used were as follows:
β-Actin, F: CTg ACT gAC TAC CTC ATG Aag ATC CT, R: CTT AAT gTC Acg CAC gAT TTC C; Acetyl-CoA Carboxylase α (ACCα, ACAC), F: CTT ggg ggC ATC CAg ATT AT, R: Agg ATC ATA Tgg ggC CTT Tg; CCAAT/enhancer binding protein (C/EBP), alpha (C/EBPα), F: TTA CAA CAg gCC Agg TTT CC, R: AAC TCC AgT CCC TCT ggg AT; Fatty Acid Synthase (FAS, FASN) F: CTC TgA TCA gTg gCC TCC TC, R: TgC TgC AgT TTg gTC TgA AC; Peroxisome Proliferator-Activated Receptor Gamma, (PPARγ), F: CCC Tgg CAA AgC ATT TgT AT, R: gAA ACT ggC ACC CTT gAA AA; Steroyl-CoA Desaturase (SCD-1) F: gAA CTT ACA Agg CTC ggC Tg, R: AgA CAT gTC CAg TTT TCC gC.
Name |
Final Conc. |
|
DMSO |
0.1%v/v |
|
SCE (OmniLean) |
12.5uM |
|
SCE (OLSI40050) |
12.5uM |
|
SCE (OLSI40050) |
12.5uM |
In vivo model-experimental design: Fifty-six (56) male C57BL/6 mice were purchased from Charles River, at the age of 5 weeks. The mice were housed individually in mouse cages in a temperature-controlled room with a 12-hour light and dark cycle and free access to regular rodent chow and water. After a week of adaptation, the mice were divided into 3 groups with 8 mice in each group. All mice were fed a high-fat diet (60% energy from fat) to induce obesity. Three groups were gavaged with low (100mg/kg/d) and high (500mg/kg/d) doses of SCE suspending in 0.5% carboxymethyl cellulose, respectively for 7.5 weeks (52 days exactly). One group was used as the obese control and gavaged with the vehicle control. Body weights were obtained daily and food intake was recorded thrice every week. Fecal samples were collected during weeks 6 and 7 by placing mice in metabolic cages for 3 days.
Sample collection: At the end of the study, the mice were anaesthetized and blood samples were collected by cardiac puncture into serum tubes containing clot-activator and placed on ice until centrifugation for serum. Liver and epididymal fat pads were collected, rinsed with phosphate-buffered saline, and weighed. Liver, stomach, muscle, and adipose tissues were collected, frozen immediately in liquid nitrogen and stored, together with serum, at -80°C.
Serum lipid analysis: Serum total cholesterol and triacylglycerols were analyzed using the standard enzymatic method on a Pointe-180 chemical analyzer. HDL was analyzed using the same method as for total cholesterol after precipitating the non-HDL cholesterol. The non-HDL cholesterol was calculated by subtracting HDL-cholesterol from total cholesterol.
Analysis of fecal crude lipids: Crude lipids were extracted from the feces (not dried) collected over 48 hr, using a modified Folch method. Briefly, fecal samples were weighed into glass culture tubes and homogenized in presence of methanol. After 15min in a shaking water bath, the tubes were cooled to the room temperature and hexane: chloroform mix (4:1, v/v) was added. After shaking for 10min, 0.88% NaCl solution was added. The tubes were vortexed and centrifuged. The supernatant was collected into clean glass tubes. The extraction was repeated twice and the supernatants were pooled. The supernatant was then dried up and the total crude lipids were used to calculate fat excretion rate (% of the total fat consumed through the diet over the 48hr fecal collection period).
Biochemical biomarkers analyses: Following the animal study, the effect of SCE treatment was assessed in the serum on a series of obesity-related biomarkers. Free fatty acids, glycerol, and obesity-related hormonal assays (ghrelin, adiponectin, leptin, insulin, glucose and GLP) were assessed.
Glycerol and free fatty acid assays: The concentration of glycerol and free fatty acids in serum was assessed using glycerol and free fatty acid assay kits (Sigma). The respective concentrations were determined by a coupled enzyme assay, resulting in a colorimetric (570nm) product proportional to the glycerol or free fatty acid present.
Ghrelin, adiponectin, leptin, insulin, and glp-1 assays: The levels of ghrelin, adiponectin, leptin, and insulin in the serum were determined by sandwich ELISA (Millipore or Mercodia). Proteins levels were measured spectrophotometrically as per each ELISA protocol. The absorbance is directly proportional to the amount of captured antigen in the unknown sample, thus the concentration could be derived by interpolation from a standard curve. The levels of GLP-1 in the serum were determined by BioPlex Pro technology (BioRad).
Histological testing: At the end of study, kidneys, liver, spleen, thymus, heart, lungs, stomach, small and large intestines, skeletal muscle and epididymal fat of mice in the obese control and SCE were harvested immediately after blood collection under anesthesia and fixed in 10% neutral buffered formalin for more than 48hours. Appropriately trimmed (2-5mm thickness) tissues were processed, embedded with paraffin, 4-5 micron sections were cut and stained with haematoxylin and eosin stain.
Statistical analysis: Data were analyzed using one-way ANOVA with repeated measures. Differences between treatments means were determined by pair wise comparisons using the least squares means test, where p <0.05 was considered significant.
In vitro models
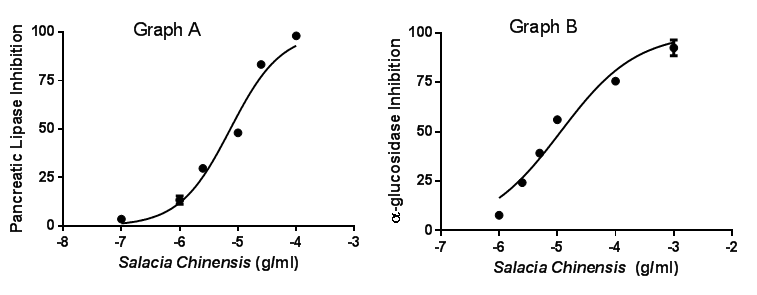
Only Salacia chinensis extract (SCE) showed a significant inhibitory effect on α-glucosidase. IC50 for SCE were 3.47µg/ml compared to acarbose (IC50:19µg/ml). SCE displayed a significant inhibitory effect against pancreatic lipase, compared to orlistat (Figure 1). SCE inhibited HMG-C0A only at higher concentration (1mg/ml) (Figure 2). SCE displayed a ratio higher that 1.40, suggesting that it enhances lipolysis in 3T3L1 cells (Figure 3). SCE induced a significant increase in the phosphorylation of hormone sensitive-lipase, almost at the same extent than the positive control, isoproterenol, confirming the lipolysis effect of SCE shown by the increase of glycerol (Table 1). SCE did have any significant effect on adipogenesis.
Samples |
Phospho-HSL |
Total HSL |
Ratio Phospho-HSL/Total HSL |
Non-Treated |
1.00 |
1.00 |
1.00 |
Salacia Chinensis |
2.43 |
2.14 |
1.14 |
Isoproterenol |
2.63 |
2.60 |
1.01 |
Table 1 Salacia Chinensis Extract (SCE) ↑ Ratio of Phospho HSL/Total HSL
Western blot densitometric analysis of HSL and Phospho-HSL isolated from adipocytes lysates.
HSL, Hormone Sensitive Lipase.
Nutrigenomics
SCE dramatically increased the expression of C/EBPα. In contrast, the gene expression pattern of SCE is predicted to decrease fatty acid synthesis (Z-score 1.9, decreased expression of FASN, ACC, PPARG and SCD-1, Figure 4). However, there are unique modes of regulation of C/EBP that may be explored in future studies.
In vivo model
As the experiments were carried out for a period of 7.5 (52 days), the body weight and food intake were reported for up to 7 weeks. Other results, except for fecal fat excretion, were obtained at the end of the study (after 52 days of treatment). There were no significant treatment effects on liver weight, epididymal fat weight as well as their relative weights (% of the body weight), whereas the effect on epididymal fat weight reached a marginal significance (Table 2).
Treatment |
FE (g/100g) |
Liver (g) |
% of Body Weight |
Epididymal Fat (g) |
% of Body Weight |
OBC |
10.8±0.6 |
1.10±0.08 |
3.06±0.24 |
2.56±0.19 |
7.08±0.44 |
Salacia Chinensis -L |
9.5±0.5 |
0.96±0.07 |
2.83±0.20 |
2.44±0.12 |
7.06±0.25 |
Salacia Chinensis -H |
6.4±1.3# |
1.04±0.08 |
3.41±0.15 |
1.94±0.28 |
6.15±0.68 |
Table 2 Salacia Chinensis Extract (SCE) ↓food efficiency and ↓ epididymal fat weight
Data were analyzed using one-way ANOVA. Differences between treatments means were determined by pairwise comparisons using the least squares means test, where p <0.05 was considered significant. Results are presented as values with their standard errors (n=7-8). #different from OBC group, p = 0.0163.
FE (Food efficiency), which was calculated as per g of weight gain over 100 g of diet consumed. Data were analyzed using one-way ANOVA. Results are presented as values with their standard errors (n=7-8). #different from OBC, p <0.05.
SCE at higher concentration significantly decreased significantly serum glycerol levels (by 69%) and free fatty acids levels (by 77%) compared to control group. Percent change in glycerol and free fatty acid levels compared to OP control. Negative values denote a decrease in glycerol or free fatty acid levels. Positive values denote an increase in glycerol or free fatty acid levels.
SCE reduced glycerol level, indicating lipolysis may not be main reason for preventing weight gain. Treatment with SCE -high dose decreased by 20% leptin levels and by 21% insulin levels and increases by 16% adiponectin and by 15% ghrelin but did not affect glucose or GLP-1 levels SCE low dose was more effective on GLP1 and increases by 22% serum GLP level (Table 3).
Group |
Ghrelin |
Glycerol Assay |
Free Fatty Acid Assay |
Adiponectin |
Leptin |
Insulin |
Glucose |
GLP-1 |
SCE -L |
12.7 |
0.17 |
22 |
12.7 |
-1.6 |
4.1 |
1 |
22.7 |
SCE -H |
15.1 |
-69 |
-77 |
15.8 |
-20.4 |
-21.0 |
1.4 |
11.1 |
Table 3 Effect of Salacia Chinensis Extracts on Ghrelin, Glycerol and FFA, Adiponectin, Leptin, Insulin, and GLP-1 Levels in Serum of Obese Mice
Salacia Chinensis Extract (SCE); L: Low; H: High; GLP-1: Glucagon-Like Peptide-1
No treatment related changes were observed. Even though incidences of perivascular lymphocytes aggregation in lungs are high in test compound treated groups, these minimal to mild changes are often seen as back ground lesions possibly associated with unknown antigenic stimulation or subclinical infections, therefore not considered as treatment related findings. Diffuse microvesicular vacuolation of liver could be associated with high fat intake. Microgranuloma in liver consists of small numbers of lymphocytes and neutrophils cells that surround and replace few hepatocytes. These are commonly observed in mouse liver and considered as a background change. A single incidence of stomach lesion is interpreted as an incidental finding (Table 4).
Organ |
Histopathology Changes |
No of Incidences |
OBC |
SR-H |
Liver |
Microvesicular Vacuolation |
4/8 |
2/8 |
Microgranuloma |
6/8 |
6/8 |
|
Lungs |
Perivascular Lymphocytes Aggregation |
- |
3/7 |
Stomach Glandular |
Submucosal Neutrophilic Infiltration |
1/8 |
- |
Table 4 Summary of histopathological testing
SCE at the dose of 500mg/kg/d significantly slowed body weight gain, reduced food intake, decreased fat pads mass and increased fecal fat excretion suggesting one of the major effects of SCE reducing fat absorption. These in vivo findings are in concordance with our in vitro results where SCE has a strong inhibitory effect on pancreatic lipase activity, compared to orlistat (IC50 were respectively 10.12µg/ml for SCE and 0.53µg/ml for orlistat). Alpha-glucosidase inhibitory effect has been identified as the primary activity responsible for its hypo-glycemic effect. The intestinal enzymes, alpha-glucosidase and alpha-amylase break down starches, dextrin, maltose and sucrose into readily absorbable monosaccharides within the small intestine. Inhibition of these enzymes would cause delay in the absorption of glucose and help attenuate the postprandial glucose surges in diabetic individuals. This mechanism is currently being clinically used in alpha-glucosidase inhibitors like Acarbose.17 Hence SCE would be expected to reduce the postprandial hyperglycaemia and hyperinsulinaemia by inhibition of poly and oligo saccharide digestion. Pancreatic lipase activity is a critical enzyme for the digestion of dietary fat and hence believed to contribute towards weight reduction.18 Thus inhibition of pancreatic lipase activity in the small intestine is believed to be the main mechanism by which postprandial hyperlipidaemia is attenuated by the Salacia root. Adipocytes, has long been regarded as a tissue whose primary effect is the storage of excess energy. Some of the species from genus Salacia have been used for centuries in Ayurvedic medicine for the oral treatment of diabetes.19-21 The extract from Salacia species has traditionally been consumed as a tea, i.e., the root is boiled in water, filtered, and consumed. Currently, extracts of Salacia are consumed in commercial foods and food supplements in Japan for diabetes and obesity.15 Yoshikawa et al.,22 isolated active components of a Salacia extract and concluded that their mode of action was the inhibition of α-glucosidase enzymes. Extracts of various Salacia species have been shown to inhibit the activity of intestinal TLC α-glucosidases.23 Several investigators have attempted to isolate active constituents from Salacia extracts,22,24,25 that have been shown to inhibit the action of the small intestinal enzymes sucrase, maltase, and isomaltase.23
Recent evidence suggests that it has endocrine function regulating glucose and lipid metabolism through active secretion of various adipocytokines [26]. In a placebo controlled crossover trial, twenty treatment naïve patients with type 2 diabetes, divided into 2 groups were given SCE extract (240mg/day) or placebo for a period of 6 weeks. The subjects were then crossed over and the experiment repeated. The results indicated significant reductions in fasting plasma glucose, HbA1C and Body Mass index (BMI) in the treatment groups.27
These results suggest that SCE is a potential extract that can prevent obesity. Obesity is often associated with elevated serum free fatty acids (FFA), because of;
Plasma FFA can easily enter cells where they are either oxidized to generate energy in the form of ATP or esterified for storage as triglycerides. Moreover FFA has been shown to interfere with insulin stimulation of glucose transport leading to insulin resistance.28
Our in vitro studies showed that SCE induced lipolysis in adipocytes when added for 24h. We demonstrated SCE treatment enhanced phosphorylation of the hormone-sensitive lipase which hydrolyzes the triglycerides into non esterified fatty acids and glycerol. The increased glycerol levels in adipocytes treated with SCE were almost at the same extent that the positive control: isoproterenol, confirming that acute treatment with Salacia Chinensis enhance lipolysis.
Chronic treatment with SCE high dose showed a weight lowering effect, prevented body mass gain and decreased fat pad mass. The decreased in adipose tissue reduced serum levels of FFA and glycerol. We did not observe any significant increase in free fatty acids or glycerol levels, when SCE was administrated chronically. Previous data showed that lipolysis in vivo and the increase in free fatty acids and glycerol occur only at short time after administration of the extract (1 to 4 hours); in our model the serum was taken 24h after gavage, The release of free fatty acids in the blood stream may be occurring after administration of the extract, but the turnover of free fatty acids is rapid and free fatty acids seem to be metabolized very quickly. Current study results are in concordance with a previous study,29 where the effect of an aqueous extract of the same herbal compound has been investigated on high fat diet mice model. The water extract inhibited weight gain at the same extent that our ethanolic extract.
In order to find the relationship between the weight loss effect of SCE high dose and appetite, we examined the serum levels of leptin and ghrelin. SCE high dose secreted significantly less leptin compared to control group. Generally leptin is an adipocyte derived hormone secreted by fat tissues and controlled by neuropeptide Y from the thalamus, which reduces food intake and suppress weight gain. When it secretion is excessive, it accelerates lipolysis and increase energy use. Our study showed a slight decrease in leptin levels that cannot be associated to the metabolic changes observed in SCE group, the decrease in leptin levels observed can only be related to the decrease in fat mass observed with SCE treatment.
In contrast to the appetite suppressing effect of leptin, ghrelin is secreted by the stomach and increase appetite. It has been shown that ghrelin can be controlled by the body energy level and it levels increases during hunger and decrease when food is eaten. The animals used were fasting overnight and that may explain the elevation of ghrelin level in serum that we observed.
Adiponectin, another adipocyte-derived hormone plays an important role in the regulation of energy homeostasis and insulin sensitivity. Yamauchi et al.,30 showed that adiponectin decreases insulin resistance by decreasing triglyceride content in muscle and liver in obese mice. This effect results from increased expression of molecules involved in both fatty-acid combustion and energy dissipation in muscle.31 Other authors confirmed that adiponectin increases fatty acid oxidation in muscle and causes weight loss in mice.32 Our results showed that treatment with SCE showed an increase in adiponectin that protected Salacia chinensis animal group from body weight gain and from insulin-resistance. This amelioration of insulin resistance is associated with an increase of fatty acid oxidation and a decrease of insulin level in serum. These results suggest that adiponectin may be another mechanism involved in the prevention of weight gain and fat absorption by inducing fatty acid oxidation.
GLP-1 is a gastrointestinal hormone that has been also assessed; it has a role in controlling appetite and energy intake. SCE high dose treatment increases slightly GLP1 levels but not significantly, this effect can be positively correlated with the 17% decrease in food intake observed in SCE high dose treated group. These results suggest that inhibiting food intake may also one of the mechanisms of action of SCE. More studies focused on biomarkers of satiety need to be performed to confirm this hypothesis.
In conclusion, SCE activity is basically attributed to the inhibitory activity of intestinal enzymes (alpha-glucosidase and alpha-amylase). Inhibition of intestinal enzymes delays glucose absorption into the blood and suppresses postprandial hyperglycemia, resulting in improved glycemic control. Further it modulates cholesterol metabolism and improves antioxidant system. SCE at the dose of 500mg/kg/d is a potential bioactive compound that can be used as a preventive natural product that slows body weight gain by controlling fat absorption via lipid metabolizing enzymes and controlling food intake. Further research in humans need to be tested to investigate the mechanisms of action of Salacia Chinensis extract (SCE).
Authors are grateful to OmniActive Health Technologies Ltd., India for funding studies and Indigo biosciences, PA, USA.
This study was supported by OmniActive Health Technologies Ltd. India.
All authors are involved in study design, data interpretation, manuscript preparation and editing: KG, VS, JD and VJ. All authors read and approved the final manuscript.
The authors declare that they are scientists and employees of OmniActive Health Technologies.
This study was approved by the Animal Ethics Committee of the NRC- Canada.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.