eISSN: 2473-0815


Functional islet beta-cell tumours represent 1%-2% of all pancreatic neoplasms. Diagnosing this type of tumour is often challenging because they present unspecific clinical features overlapping more common syndromes. Diagnosis involves expensive testing, so, establishing Whipple´s triad and excluding causes of exogenous hyperinsulinemia is crucial before initiating investigation. During the investigation, identification of hypoglycaemia associated with inappropriately high levels of insulin and C-peptide should prompt the exclusion of rare causes of hypoglycaemia, including pancreatic islet-cells disease.
Surgical resection of pancreatic islet-cells adenoma is the primary treatment modality so, accurate localization of the tumour is important, as it enables more conservative intervention. In this paper, we describe a rare case of hypoglycaemia associated with endogenous hyperinsulinism and review important aspects of the diagnosis and treatment.
Keywords: proinsulinoma, hypoglycemia, islet beta-cells tumour, hyperinsulinism
Functional islet beta-cells tumours represent 1%-2% of all pancreatic neoplasms. Diagnosing this type of tumour is often challenging because they present unspecific clinical features overlapping more common syndromes. Initially, autonomic symptoms are predominant during the hypoglycaemic episodes and later are followed by neuroglycopenic symptoms. Frequency and severity increases over time and syndrome of hypoglycaemia unawareness can be acquired as diminished counter regulatory hormone responses and adrenergic sensitivity establishes. Because of non-specific and insidious nature, the time between onset of symptoms and diagnosis is about 3 to 5 years.
In adults, hypoglycaemia is usually associated with an easily identifiable factor, such as known disease; medication or deficient counter regulatory insulin action hormones. However, in apparently healthy adults, other less common causes should be investigated. Investigation involves expensive testing, so, establishing Whipple´s triad (plasma glucose less than 54mg/dL, with symptoms that reverse after administration of glucose) and excluding causes of exogenous hyperinsulinemia is crucial.
During an episode of spontaneous hypoglycaemia or fasting test (12 or 72 hours) the following findings suggest endogenous hyperinsulinemic hypoglycaemia: blood glucose <55mg/dL during a symptomatic period, associated with insulin ≥3 IU/mL or 18pmol/L (ICMA) (>6 IU/ml or 43pmol/l, (IRMA), C-peptide ≥200pmol/L or 0.60ng/ml, proinsulin ≥5pmol/L. Additionally, anti-insulin antibodies and antibody anti-receptor insulin should be dosed.1
In this paper, we describe a rare case of hypoglycaemia associated with endogenous hyperinsulinism, whose cause is uncommon in clinical practice, and review important aspects of the diagnosis and treatment.
A 47-year-old woman was sent to the endocrinology department for evaluation of suspected spontaneous hypoglycaemia. She wasn´t taking any medications and reported no other medical problems. Her profession wasn´t related to health care. For 2 years she had adrenergic/neuroglycopenic symptoms that relieved rapidly after ingestion of simple carbohydrate-containing food. She had gained 20kg/2 years. Symptoms aggravated with time, with weekly frequency and incapacitation for work in last 6 months.
Fractionated-diet, excluding simple carbohydrates, failed to improve symptoms. The patient was taught how to use glucose meter to measure capillary blood glucose (BMT) and instructed to do a diary, that include meals content and BMT during symptoms. The diary revealed hypoglycaemias with values under 50mg/dL associated with neuroglycopenia and without a predictable pattern. A mixed meal test was programed at the Day Care Hospital, that wasn´t carried out because the patient presented at 9 p.m. with neuroglycopenia, after 13 hours of fast.
Laboratorial investigation confirmed endogenous “hyperinsulinism” (Figure 1): venous glucose-37mg/dL, unsuppressed peptide C (2.3ng/mL), Serum-hydroxybutyrate levels at lower end of normal range (0.07mmol/L), and after administration of 1mg of glucagon, glucose levels increased 35mg/dl. Autoimmunity was negative as were serum sulfonylurea levels. Insulinemia dropped to levels within normal range (3.6uUI/mL), proinsulinemia was nine times the normal range (47.3pmol/L). So we concluded that endogenous hyperinsulinism was secondary to autonomous proinsulin production (“pro-insulinoma”).
The following exams were performed on the patient:
The patient started treatment with diazoxide while waiting for surgery. She developed malleolar edema during treatment.
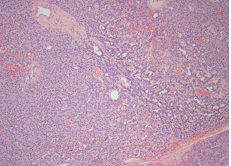
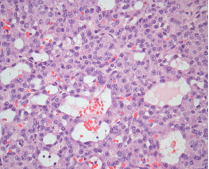
In September of 2014, she underwent a tumour enucleation, without complications (Figure 4). In the postoperative period the patient resolved symptoms and hypoglycaemia did not recur. The histopathological study confirmed a low-grade neuroendocrine tumour with high reactivity to a-chromogranin and synaptophysin (Figure 5A-D). Seven months post-surgery she didn’t present hyperglycaemia or diabetes mellitus.
Functional islet beta-cell tumours are a common cause of endogenous hyperinsulinemia. It has an incidence of 2-4 individuals/million/year and can occur sporadically or hereditarily in the context of endocrine neoplasia syndrome Multiple 1 (MEN 1) or von Hippel Lindau Disease.2,3
Usually, functional islet beta-cells tumours produce simultaneously insulin and proinsulin. Functional islet beta-cells tumours producing only proinsulin, like the one described in this clinical case, are rare.4-6
Although the circulating proinsulin has only 20% of the bioactivity compared with insulin, during
a fasting or period of high metabolic activity, the progressive decrease in blood glucose associated with failure in reducing proinsulin production results in hypoglycaemia.4 In our case, the patient reported symptoms suggestive of hypoglycaemia without a predictable pattern.
In the investigation of a suspected functional islet beta-cells tumour is important to note the type of test used to insulin dosage since, while some assays measure only the intact insulin molecule, other simultaneously measure proinsulin and C-peptide (40-80% cross-reaction between proinsulin and insulin).4,6,7 Currently, most commonly used tests immunoradiometric (IRMA) or imunoquimioluminescentes (ICMA) which do not cross-react with proinsulin. Thus, in cases of suspected endogenous hyperinsulinemia it is critical specifically order proinsulin´s dosage, as we did in the case described; otherwise there is the risk of delay or failure diagnosis. Tumour localization is also challenging. Imaging studies should only take place once the biochemical diagnosis has been established. Endoscopic ultrasound is the most sensitive and specific method.
In conclusion, endogenous hyperinsulinemia is a challenging diagnosis. The work-up of fasting hypoglycaemia is crucial because a wrong diagnosis can lead to unnecessary pancreatectomy or a missed pancreatic tumour. Highly specific serum insulin assay can difficult the diagnosis. As in this case, an enucleative approach minimizes the risk of developing post-operative diabetes, leading to a favourable prognosis.
None.
The author declares there are no conflicts of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.