eISSN: 2473-0815


Review Article Volume 2 Issue 4
Department of Endocrinology, Gulf Medical University, UAE
Correspondence: Saadi AlJadir, Department of Endocrinology, Gulf Medical University, PO Box 3243, Fujairah, UAE
Received: July 27, 2015 | Published: November 19, 2015
Citation: AlJadir S. Insulinoma: literature’s review (part 2). Endocrinol Metab Int J. 2015;2(4):152-164. DOI: 10.15406/emij.2015.02.00031
Insulinoma is rare a tumor of the islet cell of the pancreas that produces excessive amounts of insulin, inappropriately to plasma glucose levels. The average age of onset 40-50 years. The typical symptoms that patients complain about are related to the development of hypoglycemia. Patients with insulinoma usually develop neuroglycopenic symptoms (confusion, headache, visual difficulties, irrational behavior and extremely, coma) particularly with exercise or fasting. Severe hypoglycemia may result in seizures, coma, and eventually neurological damage. Symptoms resulting from the catecholaminergic response to hypoglycemia (i.e. tremulousness, palpitations, tachycardia, sweating, hunger, anxiety) are not uncommon. Weight gain is sometimes observed and not uncommonly the patient might become massively obese. Insulinomas are small in 90% <2cm, solitary> 90%, and 5-10% malignant. They are almost invariably occur in the pancreas and distributed equally in head, body and tail. Insulinoma should be suspected in all patients with hypoglycemia, especially those with attacks provoked by fasting or with a family history of MEN-1.The diagnosis of an insulinoma is usually made biochemically with low blood glucose, elevated insulin, C-peptide levels and proinsulin, by a 72-hour fasting under close supervision, and confirmed by localizing the tumor by computed tomography, MRI, or transabdominal and endoscopic ultrasonography. Sometimes detection of lesions might be challenging and ancillary tests are occasionally required .The definitive treatment is surgical removal of the tumor, and laparoscopic surgery for localized lesions is increasingly reported. Medical treatment is reserved for unresectable tumors, preoperative preparation or for unsuitable candidates for surgery.
Keywords: neuroendocrine, hypoglycemia, men-1*, islet cell tumor, whipple’s triad, Insulinoma, fasting test, mri, diazoxide
MEN-1*, *multiple endocrine neoplasia type 1
Basically the treatment of choice for patient of insulinoma is the surgical removal of the tumor, the first step is biochemical confirmations of tumor existence and appropriately other causes of hypoglycemia have been excluded (Table 1) & (Table 2), Although biochemical diagnosis of endogenous hyperinsulinemic hypoglycemia is straightforward, surgical removal of an insulinoma is hampered by difficulties to localize it using conventional radiological procedures (endosonography, MRI, CT-imaging techniques).1–8
Therapeutic modality |
Indications |
Contraindications |
Somatostatin analogues |
Symptom control, growth stabilization |
Octreoscan-negative patients |
Interferon-alpha |
Symptom control, growth stabilization |
Leucopenia |
Debulking |
Symptom control |
General contraindications for surgery |
Hemihepatectomy, segmental resections |
Curative/debulking – symptom control |
General contraindications for liver surgery |
Local ablative therapy (RFA, LITT) |
Limited number of liver metastases |
Multiple (>10) liver metastases, or 0 > 4–5 cm |
Liver metastases |
(<8–10), 0 < 4–5 cm, curative, symptom control |
|
Embolization/chemoembolization liver |
Liver metastases (>70% tumour load)/symptom control |
Occluded portal vein, biliary |
Radiolabelled microspheres (SIRT) liver |
Liver metastases (>70% tumour load)/symptom control |
Occluded portal vein, liver failure, lung shunting, |
Liver transplantation |
Liver metastases/symptom control |
Extra hepatic tumor (except in the case of |
Systemic chemotherapy |
Metastatic disease, symptom control |
Low performance score, cytopaenias, liver or |
PRRT |
Metastatic disease, symptom control |
111In-pentetreotide scan-negative lesions, |
131I-MIBG |
Metastatic disease, symptom control |
Absent uptake of 123I-MIBG, cytopaenias, liver or |
Table 1 Therapeutic modalities for metastatic Insulinoma
Abbreviations: RFA, radiofrequency ablation; LITT, laser-induced thermotherapy; SIRT, selective internal radiotherapy; PRRT, peptide receptor radiotherapy; MIBG, metaiodobenzylguanidine.
Preoperative procedures
Non-invasive imaging techniques like ultrasonography (US), computerized tomography (CT) and magnetic resonance imaging (MRI) can be helpful in localization of tumors larger than 1cm but fail to detect small insulinomas. Invasive techniques like selective arteriography, transhepatic portal-venous sampling (THPVS), endoscopic US (EUS), and intraoperative US have better results in localization of insulinomas, but they have certain disadvantages. Some authors believe that the preoperative localization can be bypassed, because relatively high percentage of insulinoma can be identified by an experienced surgeon during the operation. However, some studies showed that in 10%-27% of patients without preoperative localization the insulinoma could not be detected intraoperatively. As a result, preoperative localization is necessary for the successful surgical treatment of insulinoma. Localization of the tumor has helped reduce the degree of pancreatic trauma intraoperatively and determine the operation success.9–13
Preoperative transabdominal US
Like several abdominal disorders, transabdominal US yields the widest range of success and failure of all preoperative localization tests. It is non-invasive, free of radiation exposure, readily available, relatively inexpensive, and anatomically precise. Key major drawbacks include its extreme dependence on operator expertise and limitations based on the patient's habitus that is usually unfavorable since many insulinoma patients are overweight or obese. Overall sensitivity of this test is 27% in recent series.14–22
CT scan
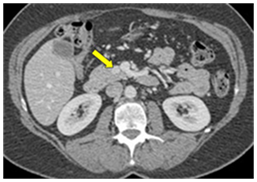
CT scan has many advantages since it can be easily performed, is non-invasive and the images are easily interpretable for the surgeon. Insulinoma typically appear as well-defined, rounded, homogeneously masses of the pancreas. Because the majority of insulinoma are usually smaller than 2cm, dynamic CT scan should be performed; the sensitivity of the dynamic CT scan in the detection of insulinoma ranges from 30% to 66%. Dual-phase contrast spiral CT scan is more sensitive than other noninvasive imaging studies. In a group of seven patients with tumors that were biochemically proven but not previously located by ultrasonography, CT scan, or magnetic resonance imaging, six of seven tumors ranging from 6 to 18 mm were detected by dual-phase spiral CT scan. Atypical CT scan imaging of insulinoma includes hypoattenuating masses on enhanced CT or intra-arterial dynamic CT, cystic masses, and calcified masses (Figure 1–3).26–30
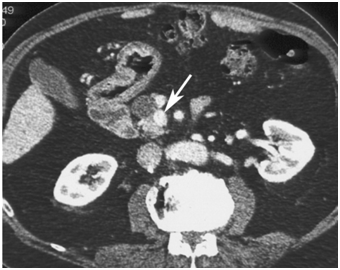
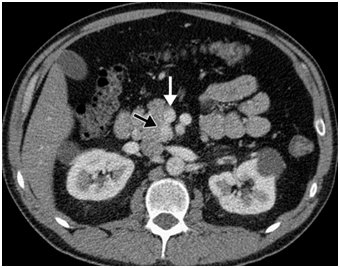
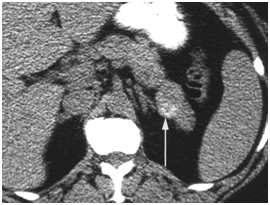
MRI
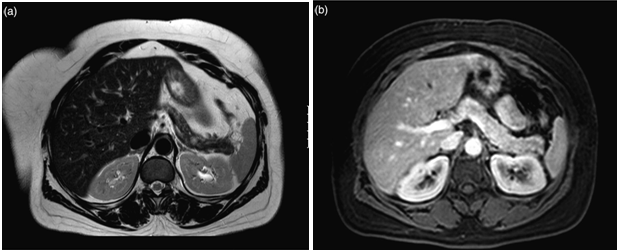
The latest studies have demonstrated that MRI is superior to other preoperative imaging techniques in identifying small pancreatic insulinoma. Its sensitivity ranges from 85% to 95%, in the detection of insulinoma and the determination of the presence of metastases. Using conventional sequences, small insulinoma usually have a low signal on T1-weighted sequences and a high signal on T2-weighted sequences. Some insulinomas containing fibrous tissue may show low signal intensity on both T1- and T2-weighted images. An improvement in MRI technique is the use of diffusion weighted MRI (DWI-MRI) for abdominal imaging. DWI is an MRI technique that detects changes in the molecular diffusion of water in biologic tissues and the valuable role of DWI in the detection of pancreatic tumors has been reported in several studies (Figure 4A), (Figure 4B) & (Figure 5).33–36
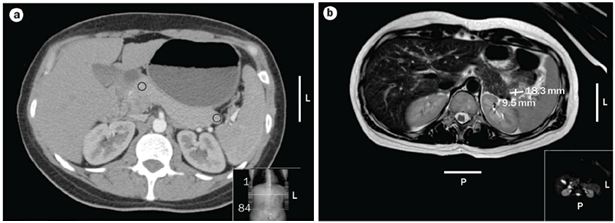
Positron emission tomography (PET)
The results of 18 F-fluorodeoxyglucose (18 F-FDG) PET imaging for insulinomas are not very promising, maybe due to the low proliferative potential of these tumor cells. Positive results have been shown using 11 C-5-hydroxy-Ltryptophan (C-5-HTP), 18-3, 4-Dihydroxy-6-fluoro-DLphenylananine (F-DOPA), and 67 Ga-DOTA-DPhe 1-Tyr 3-octreotide (67 Ga-DOTATOC) due to selective uptake in tumor tissue compared to surrounding tissue. These techniques produce very good tumor visibility and can be used for the examination of both the thorax and abdomen. However, lack of general availability of PET scanning and high cost limit its use.9,37
Angiography
Angiography combined with calcium stimulation and THPVS was previously considered the gold standard of insulinoma localization, with a sensitivity ranging from 87.5% to 100%. THPVS is the most accurate preoperative tool for the localization of insulinomas. However, this method is both invasive and technically demanding, since it requires intra-arterial and intravenous catheterization and should be employed only upon high suspicion for insulinoma and failure of all non-invasive imaging tests to detect the lesion.38–40
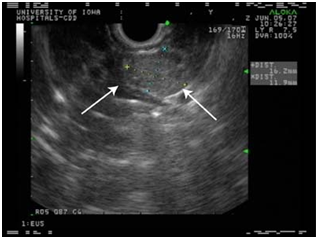
Endoscopic ultrasound
EUS allows the positioning of a high frequency (7.5-10 MHz) transducer in close proximity to the pancreas and lesions as small as 5 mm as well as tumors located in the bowel can be detected.
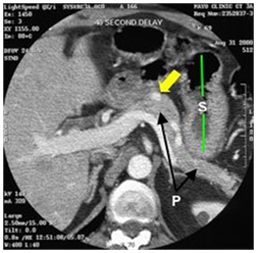
Although there is a potential "blind-spot" at the splenic hilum, a high sensitivity has been obtained. A recent single-centre prospective study showed a sensitivity of 93% and a specificity of 95% in localization of intra-pancreatic lesions. EUS could detect all tumors visualized by every other conventional technique, thus questioning the need for the rest imaging modalities (Figure 6–8).44–48
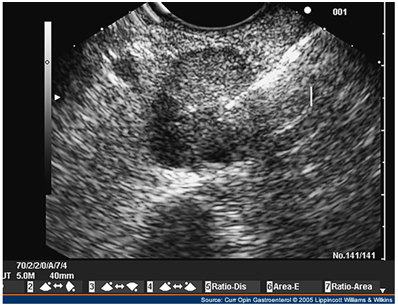
Intraoperative ultrasound
IOUS has been very useful in localizing small tumors. Additionally, it demonstrates the relevant operative anatomy, defining the relationship of the tumor to the pancreatic and bile duct and adjacent blood vessels. Intraoperative localization techniques, which include both careful palpation of the pancreas and the use of IOUS, remain the most reliable way to localize insulinomas and determine the indicated surgical procedure (enucleation versus middle pancreatectomy). Moreover, it is mandatory in patients, where multiple lesions are suspected. Laparoscopic IOUS in experienced hands can identify over 85% of insulinomas (Figure 9).50,51

Somatostatin receptor scintigraphy
The main indication for 111Indium-pentetreotide scintigraphy is the imaging of neuroendocrine tumors, like gastroenteropancreatic (GEP) tumors and sympatho-adrenal system tumors, which usually have a high expression of somatostatin receptors. Because not all insulinomas express somatostatin receptor type 2, 111In-pentetreotide scintigraphy is positive in only 46% of benign Insulinomas.52,53
Recent test
In vitro data suggest that human insulinoma cells exhibit a high density of GLP-1R. 111In-exendin-4 is a 111In labeled GLP-1R agonist that binds with high affinity to GLP-1R and may be helpful in localizing benign, small insulinomas, both preoperatively and, with the use of a gamma probe intraoperatively.54–56
Medical management
It is also available for the subgroup of patients, who are unable or unwilling to undergo surgical treatment, for preoperative control of blood glucose levels or for unresectable metastatic disease.
Dietary modification
It is designed to prevent prolonged periods of fasting with dietary management. The cornerstone of medical management of insulinoma and other forms of hyperinsulinism is the diet. Not uncommonly, patients may avoid symptoms of hypoglycemia for variable periods of time by shortening the number of hours between feedings. For some, the inclusion of a bedtime (11:00 pm) snack is sufficient; for others, a midmorning and mid afternoon snacks are necessary. Although the tumor may be stimulated occasionally to secrete insulin by the ingestion of carbohydrates, it is not advisable to restrict the carbohydrate intake. More slowly absorbable forms of carbohydrates (e.g. starches, legumes, bread, and rice) generally are preferred. During hypoglycemic episodes, rapidly absorbable forms (e.g., fruit juices with added glucose or sucrose) are indicated.57
Diazoxide & natriuretic benzothiadiazines
Diazoxide (Proglycem, Hyperstat) owes its potent hyperglycemic properties to two effects; it directly inhibits the release of insulin by ß- cells through stimulation of ß2 -adrenergic receptors, and it has an extrapancreatic hyperglycemic effect, probably by inhibiting cyclic adenosine monophosphate phosphodiesterase, resulting in higher plasma levels of cyclic AMP and enhanced glycogenolysis. Because diazoxide induces the retention of sodium, edema is troublesome at higher dosages. The addition of a diuretic benzothiadiazine (e.g. trichlormethiazide) not only corrects or prevents edema but synergizes the hyperglycemic effect of diazoxide. At the doses needed to counteract the higher doses of diazoxide (e.g. 450-600mg/d), natriuretic benzothiadiazines frequently induce hypokalemia. Nausea is an additional complication at higher dosages of diazoxide, and hypertrichosis may complicate long-term treatment. These compounds have been useful to elevate blood levels of glucose into the euglycemic range if operation must be delayed for weeks or months. Patients with benign insulinomas have been managed successfully for up to 16 years with diazoxide in doses of 150 to 450mg/d in combination with trichlormethiazide in doses of 2 to 8mg/d. If they can be tolerated, higher doses may be used in patients with malignant Insulinomas.58–61
Calcium channel blockers
Theoretically, calcium channel blockers are capable of inhibiting insulin secretion. Verapamil has been used successfully to alleviate the hypoglycemia caused by an insulin-secreting pancreatic tumor in a 94-year-old woman. Verapamil and diltiazem have been used with variable results in other patients with organic hyperinsulinism.62
Propranolol
B-Adrenergic-receptor blocking drugs inhibit insulin secretion and therefore may be of value in treating organic hyperinsulinism. Only a few reports of the use of propranolol have appeared. Its use has been associated with the reduction of plasma insulin levels and with the relief of hypoglycemic attacks in patients with benign or malignant insulinoma. In a patient with a benign insulinoma, 80 mg of propranolol a day was sufficient, whereas a patient with malignant insulinoma, in whom streptozotocin was no longer effective, required 640 mg of propranolol orally per day). Because this drug can mask the adrenergic symptoms of hypoglycemia and inhibit muscle glycogenolysis, however, there is a risk of aggravating the clinical syndrome. The drug should be used with extreme caution and careful monitoring.63,64
Diphenylhydantoin
The anticonvulsive diphenylhydantoin (Epanutin, Dilantin) has been shown to inhibit the in vitro release of insulin from both the labile and storage beta-cell pools. It has been used successfully to control refractory hypoglycemia, as evidenced by normal overnight fasting glucose levels and absence of hypoglycemia during total fasting of up to 24 hours. In only one-third or less of patients with benign insulinoma, however, is the hyperglycemic effect of Dilantin of any clinical significance. Furthermore, with the dosage required, ataxia, nystagmus, hypertrophic gums, and megaloblastic anemia may be undesirable effects. Maintenance doses range from 300 to 600 mg/d65,66
Long-acting somatostatin analogues
Treatment with SSAs can improve (inhibition of insulin release) or worsen (profound suppression of counter regulatory hormones; GH and glucagon) the hypoglycemia- associated symptoms (Maton 1993). The efficacy of octreotide on hypoglycemia was assessed in a study of 17 patients with insulinoma (Vezzosi et al. 2005): in more than 50% of the patients, the drug was effective in hypoglycemia control. A positive subcutaneous short octreotide test (100 mg octreotide s.c. in fasting patients, with improvement in hypoglycemia) was a better marker of the therapeutic response compared with a pretreatment positive scintigraphy. Another report of the successful use of octreotide (Sandostatin) in prolonging the ability to fast in a patient with a benign insulinoma, as well as a similar experience in a patient with a malignant tumor. A more recent experience has shown a variety of responses not easily predictable by the clinical or biochemical profile. They have examined the effects of a long-acting octreotide analogue in seven patients with endogenous hyperinsulinism: five with proven single adenomas, one with multiple adenomas, and one with organic hyperinsulinism associated with MEN-1. In two patients, and possibly a third, octreotide prolonged the ability to fast without hypoglycemia, with variable decreases in plasma insulin concentrations. A trial of long-term administration of octreotide in one of these patients showed only short-term relief of hypoglycemia. Octreotide did not improve, or rather worsened plasma glucose levels on fasting in the other four patients. It is unlikely that octreotide will be a useful addition to the therapeutic armamentarium for treatment of organic hyperinsulinism, except in familial forms of nesidioblastosis. A study in benign and malignant insulinoma showed that somatostatin receptor subtype 4 was most frequently expressed in both benign and malignant tumors. 3 of the 6 malignant tumors, but none of the benign tumors expressed somatostatin receptor subtype 5. The other receptor subtypes were expressed in low numbers with no difference between benign and malignant tumors.67–74
Glucocorticoids
The use of glucocorticoids, which increase gluconeogenesis and cause insulin resistance, also can help to stabilize blood glucose at an acceptable level. Pharmacologic doses (prednisone, approximately 1 mg/kg) must be used.60,75
Glucagon
Glucagon may help to raise blood glucose concentrations, but it may simultaneously directly stimulate the release of insulin, it might be reserved in emergency situations with profound hypoglycemia.
Estrogen receptor blockers
Recent data suggest that estrogen and progesterone receptors are frequently expressed in insulinomas and that these receptors appear to be functional. However, whether or not estrogen receptor blockers can effectively decrease insulin levels in these patients is not known and more study required.76
Treatment of malignant Insulinoma
mTO Inhibitors
mTOR is a central regulator of protein synthesis important in cancer, including cell growth and proliferation, angiogenesis and cell metabolism. Several genetic syndromes associated with NETs involve signaling through the mTOR pathway (Figure 10). Everolimus, (Afinitor®, Novartis), is a new oral, once-daily mTOR inhibitor that blocks the mTOR pathway by binding to its intracellular receptor, FKBP-12. In preclinical studies, everolimus inhibited tumor growth in a variety of human solid tumors, both in vitro and in vivo. Everolimus has synergistic anti-tumor effects when used in combination with other anticancer agents and may have a similar effect when used in combination with a somatostatin analogue. A study of 60 patients with metastatic low- to intermediate-grade NETs (30 with primitive NETs and 30 with nonprimitive NETs) assessed the antiproliferative effect of combining everolimus with octreotide. Promising anti-tumor activity and PFS was observed in those who received a higher dose of everolimus (10 vs 5 mg/day) had slightly better partial response and PFS rates. A Phase II trial of everolimus with or without octreotide LAR in patients with advanced pancreatic NETs following chemotherapy failure (RADIANT-1) found that in those receiving everolimus monotherapy, median PFS was 9.7 months, and 89 patients (77.4%) experienced a stabilization or decrease in tumor size. By contrast, patients receiving everolimus 10 mg/day plus octreotide 30 mg/month achieved a median PFS of 16.7 months, and 38 patients (84.4%) had tumor stabilization or shrinkage. In the RADIANT-3 study, a Phase III trial in advanced pancreatic neuroendocrine tumors, 410 patients were randomized either to everolimus 10 mg/day plus best supportive care or placebo plus best supported care. The majority of the patients presented well differentiated or moderately differentiated tumors. Half of the patients in each arm had previously been treated with chemotherapy and 50% of the patients in each arm also received somatostatin analogs. Median PFS in the treatment arm was 11.4 vs 4.6 months in the placebo arm (HR: 0.35; p < 0.0001). The most common side effects in all the RADIANT trials are stomatitis followed by infections and pneumonitis. However, grade 3/4 adverse events only occurred in approximately 5% of patients and therefore everolimus should be considered a safe and well tolerated treatment in patients with neuroendocrine tumors (abstract presented at ESMO 2010). An additional advantage of everolimus in the treatment of patients with metastatic insulinomas is its capability to increase blood glucose levels.78–84
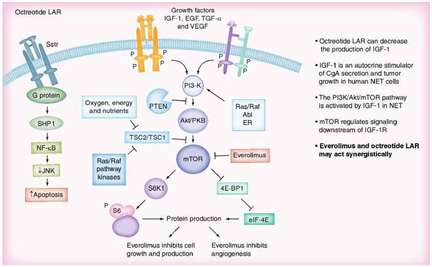
Tyrosine kinase inhibitors
Sunitinib (Sutent®, Pfizer) an oral multi-targeted tyrosine kinase inhibitor was applied in a Phase III randomized double blind trial in patients with pancreatic neuroendocrine tumors. A total of 171 patients were randomized either to sunitinib 37.5 mg/day orally or placebo. They had well-differentiated malignant pancreatic neuroendocrine tumors with disease progression in the past 12 months. Initially, 340 patients were planned but an interim analysis closed the study early. Kaplan-Meier analysis of PFS showed in the sunitinib arm a median PFS of 11.4 months versus 5.5 months in the placebo arm (HR: 0.418; p=0.0001) (Figure 4). The objective response rate was 9.3 months in the sunitinib arm and 0 months in the placebo arm. Median duration of response was 18.1 months. The most frequently reported all grade adverse events with sunitinib were diarrhea (59%), nausea (45%), asthenia (34%) and fatigue (33%). Most common grade 3 or 4 adverse events included neutropenia, hypertension and hand/foot syndrome. The drug seems to be well tolerated (ASCO abstract 2010).85,86
Chemotherapy
Single chemotherapeutic agents in midgut carcinoid tumors such as fluorouracil, dacarbazine, doxorubicin and streptozotocin were initially evaluated with little beneficial effect. As such, combination chemotherapy trials were conducted to try to improve on these results. However, there is no evidence that multi-agent regimens are any more effective; no regimen has demonstrated a response rate greater than 15% using the criterion of a 50% decrease in bi-dimensionally measurable disease. Combination of chemotherapy and IFN- therapy does not appear to improve on the results of monotherapy. However, in patients with pancreatic endocrine tumors, response rates of approximately 40% have been reported for streptozotocin in combination with other agents such as 5- fluorouracil, cisplatin or doxorubicin.Furthermore, temozolomide has demonstrated promising anti tumor effects in pancreatic NETs. A study combining temozolomide and thalidomide in 29 patients with metastatic carcinoid, pheochromocytoma or pancreatic NETs observed an objective biochemical (CgA) response rate of 40%, and a radiologic response rate of 25%; this was 45, 33 and 7% in patients with pancreatic NETs, pheochromocytomas and carcinoid tumors, respectively. The median duration of response was 13.5 months, 1-year survival was 79% and 2-year survival was 61%. The response rate seems to be related to the expression of 06-methylguanine DNA methyltransferase (MGMT). Low expression gives a higher response rate (40%) versus high expression (0%). A recent Phase II study in naive pancreatic neuroendocrine tumors treated with a combination of temozolomide plus capecitabine presented a PFS of 18 months and partial response (RECIST) in 67% of the patients.87–94
Systemic chemotherapy
Historically, streptozocin combined with doxorubicin and/or 5-fluorouracil (5-FU) has been used as chemotherapeutical regimens in patients with metastatic, progressive insulinomas. These combination treatments may reduce hormonal symptoms and result in objective tumour responses in 6-70% of patients with PNETs, including insulinomas (Table 2). Studies on the effects of chemotherapy on patients with PNETs largely differ with regard to total number of patients studied, and different objective criteria for measuring tumor response were used in the past, and generally specific information on malignant insulinomas is lacking (Table 2). Generally, patients with nonfunctioning/nonsyndromic PNETs are overrepresented in these studies. Moreover, information on antisecretory effects of chemotherapy on functioning/syndromic PNETs, and specifically on patients with metastatic insulinomas achieving normoglycaemia, is generally absent in these oncological series (Table 2). Nowadays, staging and grading systems apply and tumor responses should be scored according to RECIST (Response Evaluation Criteria In Solid Tumors) criteria. Among the most prevalent, clinically relevant side effects of these chemotherapies are cardiac, renal and/or liver toxicities, myelosuppression, mucositis, fatigue and diarrhoea. Recently, quite favorable results have been reported using first-line chemotherapy with capecitabine plus temozolomide in patients with PNETs (including insulinomas), achieving 70% objective tumor responses, a median progression-free survival (PFS) of 18 months and a 2-year survival of 92% (Table 2). Dacarbazine has been used as a second-line therapy in PNETs (Table 2). Again, specific data on insulinoma in these studies are lacking. It was also shown that the combination of concurrent and sequential chemo-radiotherapy can be an effective treatment for locally advanced PNETs (Table 2). PNET, pancreatic neuroendocrine tumour; ORR, objective response rate? Unkown (not reported). *Biological responses in 9/10 functioning tumours. ‡24 endocrine hyperfunction - not further defined. †19/21 patients with ORR and also endocrine response - not further defined.95–106
Reference |
Chemotherapy |
First line (%) |
Patients |
ORR (%) |
Patients with |
Insulinoma |
Biochemical |
Moertel |
Streptozocin/Streptozocin + 5-F-U |
98 |
42/42 |
36/63 |
8/7 |
37Æ5/88 |
? |
Rougier |
Doxorubicin + cisplatin + 5-F-U |
80 |
5 |
20 |
? |
? |
? |
Moertel |
Doxorubicin + streptozocin/chlorotocin/ |
100 |
38/33/34 |
69/30/45 |
2/3/1 |
67 |
* |
Cheng |
Doxorubicin + streptozocin |
100 |
16 |
6 |
1 |
? |
? |
Rivera |
Doxorubicin + streptozocin + 5-F-U |
83 |
12 |
54 |
? |
? |
? |
Kouvaraki |
Doxorubicin + streptozocin + 5-F-U |
94 |
83 |
39 |
3 |
? |
? |
Delaunoit |
Doxorubicin + streptozocin |
76 |
45 |
36 |
3 |
? |
† |
Turner |
Cisplatin + streptozotocin + 5-F-U |
100 |
49 |
38 |
‡ |
? |
? |
Ramanathan60 |
Dacarbazine |
56 |
50 |
34 |
? |
? |
? |
Ekeblad |
Temozolomide |
1st <<2nd |
12 |
8 |
? |
? |
? |
Kulke |
Temozolomide + thalomide |
50 |
11 |
45 |
? |
? |
? |
Strosberg |
Temozolomide + capecitabine |
100 |
30 |
70 |
2 |
? |
? |
Table 2 Chemotherapy of metastatic pancreatic neuroendocrine tumors (PNETs) including insulinomas.
Abbreviations: PNET, pancreatic neuroendocrine tumour; ORR, objective response rate; ?, Unknown (not reported). *Biological responses in 9/10 functioning tumours. ‡24 endocrine hyper function– not further defined. †19/21 patients with ORR and also endocrine response - not further defined.95–106
Radionuclide Therapy
Although the response rate of NETs to external beam radiation is limited, the introduction of systemic receptor-targeted therapy (peptide receptor radiotherapy (PRRT)) has provided beneficial effects in patients with unresectable somatostatin receptor-positive NETs. Current data suggest objective response rates of 30-40% with disease stabilization in 40% of patients. One study assessing PRRT with the radiolabeled somatostatin analog 9177Lu-DOTA0, Tyr30 octreotate in 310 patients with NETs observed complete and partial tumor remissions in 2 and 28%, respectively.] Minor tumor response (decrease in size >25 to <50%) occurred in 16% of patients. Compared with historical controls, there was a survival benefit of 40-72 months from diagnosis.107–109 A suggested algorithm for the treatment of patients with metastatic NETs, based on the author's experience, is included in Figure 11.

Surgical management
After establishing the diagnosis and the proper localization of the tumor, surgical enucleation of the tumor is the treatment of choice Preoperative localization of insulinoma improves cure rate and reduces complications, but may be challenging. The patient should be hospitalized and preoperative preparation arranged at least one day before surgery. Dextrose 10% solution is infused to avoid hypoglycemia particularly after mid-night, and diazoxide is administered on day of operation in order to reduce the need for glucose supplement and to avoid hypoglycemia. Consultation of the anesthesiologist to plan precise intraoperative blood glucose monitoring is necessary and blood glucose levels should not fall below 40-50 ml/dl. The majority of insulinomas is small and may not be easily exposed during the surgical procedure, particularly if they are not located at the anterior surface of the gland. Enucleation is the procedure of choice for all benign insulinoma. Traditionally, blind distal pancreatectomy has been considered the standard surgical procedure, when a tumor could be not visualized and/or palpated intraoperatively, but with advance in imaging this procedure had been almost abandoned. Preoperative EUS and IOUS as well as careful surgical palpation have an almost 100% sensitivity in identifying normally localized (intra-pancreatic) small insulinomas (Figures 12A), (Figure 12B) & (Figure 12C).
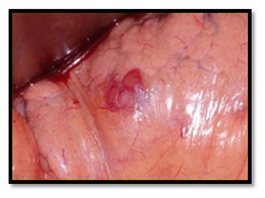
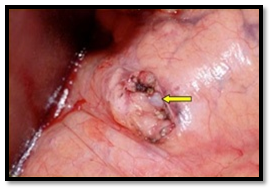
In 1996, Cushieri et al; reported the first series of laparoscopic distal pancreatectomy. Since then, few reports of laparoscopic pancreatic surgery have been published. Prior to the laparoscopic era, the division of the gastrocolic ligament was followed by careful intraoperative exploration both visually and by palpation. IOUS and surgical exploration were the ideal localizing strategy for pancreatic insulinomas. However, in the laparoscopic era, inspection and laparoscopic US are the usual localizing modality. In the absence of tactical sense, experience with the laparoscopic US technique is mandatory, accuracy of the technique can only be achieved in centers with significant number of neuroendocrine tumors.
More recently EUS-guided fine needle preoperative tattooing of insulinoma with methylene blue and laparoscopic enucleation of the tumor has recently been suggested as an alternative preoperative localization technique, a technique that mandates an experienced endoscopist and a skilled surgeon in laparoscopic and pancreatic surgery. After a successfully removal of an insulinoma the blood glucose level rapidly rises to 120-140 ml/dl, sometimes even while the patient is in the recovery room. The intravenous glucose solution should be administered for an additional 24-hour period and during that time blood glucose levels may rise up to 180-230 mg/dl. For a period of several days to several weeks the glucose levels may increase up to 200-400 mg/dl and in these cases small doses of insulin should be administered.
Surgical options might be considered in metastatic disease like:
Additional measures can be applied (Oncological & Radiological options) which are:
Post-operative complications
The most common complication of enucleation for pancreatic insulinomas is pancreatic leak. It presents either as pancreatic fistula or as an intra-abdominal collection, usually self-limited after few weeks. The use of fibrin glue has been reported to decrease the leak rate. Pancreatoduodenectomy or distal pancreatectomy, rarely indicated for malignant tumors or failed enucleation of multiple insulinomas, has a higher complication rate. Although surgical mortality in pancreatoduodenectomy is less than 5%, postoperative morbidity remains high and is approximately 50% even in large series. Open and laparoscopic distal pancreatectomy, in the absence of pancreaticojejunostomy, is followed by a minimal mortality rate, but a significant rate of pancreatic fistula, ranging from 15% to 40%.After the complete removal of the tumor, a patient with insulinoma is considered cured, but periodic follow up is important, especially for patients with malignant insulinomas and MEN-1 subjects.116,117
The combination of biphasic thin section helical CT scan and EUS has an almost 100% sensitivity in localizing insulinomas. Laparoscopic US is mandatory for intraoperative localization of these tumors. EUS-guided fine needle tattooing represents an alternative method, when laparoscopic US is not available. Laparoscopic resection of benign insulinomas is the procedure of choice, whereas pancreatectomies are reserved for the rare cases of large, potentially malignant tumors. We conclude that insulinoma is less rare than previously suspected. After successful surgical removal, the long-term risk of recurrent insulinoma is relatively high in patients with MEN I; for patients with benign disease, the long-term survival is normal.119,120
None.
The author declares that there are no conflicts of interest.

©2015 AlJadir. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.