eISSN: 2378-315X


Research Article Volume 12 Issue 3
1University of Minnesota Twin Cities, School of Public Health, USA
2University of the District of Columbia, USA
Correspondence: Adeniyi T Togun, MD, MPH, MS, PhD, University of Minnesota Twin Cities, School of Public Health, Washington DC, USA
Received: May 04, 2023 | Published: May 18, 2023
Citation: Togun ATT, Bamikole IA. Understanding risks and time to congestive heart failure following incident atrial fibrillation. Biom Biostat Int J. 2023;12(3):62-66. DOI: 10.15406/bbij.2023.12.00385
Objectives: Evaluate how different comorbidities increase 3-year risk of incident congestive heart failure (CHF) in new atrial fibrillation (AF) patients and the time from diagnosis of AF to developing incident CHF while controlling for risk factors and treating death as a competing risk event.
Methods: We utilized CMS Medicare 100% inpatient and outpatient claims data between 2013 and 2017. Patients with new AF diagnosis (without concurrent CHF at AF diagnosis) in 2014 who had 1-year continuous enrollment prior to their AF diagnosis (baseline period) and no prior diagnosis of AF or CHF in the baseline period were identified.
Results: With every 1-year increase in age of incident AF patients, 3-year risk of CHF increases by 2.6%, presence of coronary artery disease, COPD, diabetes mellitus, peripheral artery disease, and cardiomyopathy increases 3-year risk of CHF by 22.1%, 30.2%, 31.9%, 15.2%, 44.8%, and 6.3% respectively. Blacks with incident AF had 6.9% higher 3-year risk CHF than Whites. For the average aged female patient in our cohort (78.5years) with no comorbidities and death as a competing risk, we found the probability of CHF within the first 3 years after AF diagnosis to be 23.5%, 17.5% within the first 2 years, and 10% within the first year.
Conclusion: Various comorbidities affect the risk of CHF differently in incident AF patients. Cumulative risk of incident CHF appears to increase fastest within the first few years following incident AF diagnosis.
Keywords: atrial fibrillation, congestive heart failure, risk of congestive heart failure, time to congestive heart failure
AF, atrial fibrillation; CHF, congestive heart failure; US, united states; CMS, center for medicare services; ESRD, end stage renal disease; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; PAD, peripheral artery disease; MI, myocardial infarction; CCS-SAF scale, Canadian Cardiovascular Society Severity in Atrial Fibrillation scale; EHRA score, European Heart Rhythm Association score of atrial fibrillation
Atrial fibrillation (AF) is the most common type of cardiac arrythmia seen in clinical practice and increases the risk of thromboembolism, stroke, congestive heart failure (CHF), and other cardiovascular complications.1 AF and CHF often co-exist and are known risk factors for one another. Thomas J Wang et al.2 analyzed the temporal association between AF and CHF in participants of the Framingham study who had both conditions, “38% had AF first, 41% had CHF first, and 21% had both diagnosed on the same day”.2
The prevalence of AF in the United States (US) is on the rise. About 2.3 million adults in the US currently have AF, and that number is projected to more than double (5.6 million) by the year 2050.3 As the AF burden grows, related hospitalizations, health care costs, and mortality will also increase.4 Between 1991 and 2015, age adjusted hospitalization for AF increased by 1.61% yearly, years of potential life decreased by 9.8% yearly, and AF-related mortality rates increased annually.5
AF increases the risk of cardiovascular diseases like myocardial infarction, ischemic stroke and CHF.1–3 CHF is the most common cardiovascular disease complication of AF;5,6 patients with AF have 3.04 to 3.18 times the risk of congestive CHF than patients without AF.7,8 AF can lead to CHF by various mechanisms such as reducing cardiac output from rapid, inadequate, and irregular ventricular filling; loss of atrial contraction;9 fibrosis; neurohormonal activation of the renin-angiotensin-aldosterone system;10 and directly through tachycardia induced cardiomyopathy.11
In addition to the burden of AF on healthcare, patients who progress from AF to CHF constitute additional healthcare burden in the US. About 5.7 million adults in the US have CHF, and it accounts as a direct or contributing cause for one in every nine deaths.12 About 50 percent of persons who develop CHF die within the first 5 years of diagnosis.13 In addition to a high risk of death, CHF is also associated with higher health care spending and utilization. In the US, 1-2% of healthcare spending goes toward the management of CHF for a total of about $30.7 billion annually.14 CHF burden in terms of cost and adverse events like mortality remains a significant challenge to tackle, and it is therefore important to understand risk factors for CHF. Since presence of other comorbidities along with AF increase the risk of developing CHF differently, we evaluated how the presence of other comorbidities increase risk of incident 3-year CHF in AF patients.
Also, despite several advancements in the treatment of AF including catheter ablation that better maintains sinus rhythm,15 the incident rate of congestive CHF following AF has not diminished overtime.16,17 While several mechanisms behind the progression to CHF after AF are known, there is paucity of information about the timing of this progression. Hence, we also evaluate the time it takes from incident diagnosis of AF to developing incident CHF while controlling for other risk factors and death as a competing risk. Overall, the study evaluates how the presence of other comorbidities increase risk of incident 3-year CHF in in newly diagnosed AF patients and the time it takes from incident diagnosis of AF to developing incident CHF while controlling for other risk factors and death as a competing risk.
We conducted a retrospective cohort study of Medicare patients in the US using information from de-identified administrative claims.
Data source and study sample
We utilized the Center for Medicare Services (CMS) Medicare 100% inpatient and outpatient claims data during the study period of 2013 to 2017. Medicare is the public health insurance program in the United States that primarily provides health insurance for persons aged 65 and older. The study included patients with first- time AF ICD 9 or 10 diagnosis codes (without concurrent CHF) in the year 2014 who had evidence of 1-year continuous enrollment in the baseline period (1 year prior to the index diagnosis date in 2014). Patients who had a prior history of AF in the baseline as well as patients with a prior CHF diagnosis in the baseline were excluded (to exclude non-incident AF and CHF patients). Patients with end stage renal disease (ESRD) and patients younger than 65-years old were also excluded. The study was considered IRB exempt by the University of Minnesota Institutional Review Board (IRB ID: STUDY00012178) on 3/5/2021, because the data was existing and de-identified. (Figure 1)
Study measures
Patient demographic information like age, sex, and race were collected at incident AF diagnosis while baseline comorbidities like Hypertension, Coronary Artery Disease (CAD), Chronic Obstructive Pulmonary Disease (COPD), Diabetes Mellitus (DM), Peripheral Artery Disease (PAD), Myocardial Infarction (MI), chronic steroid use, and Cardiomyopathy were flagged as indicator variables using ICD 9 and 10 codes at any point during the year prior to incident AF diagnosis.
Outcome measure
3-year Congestive Heart Failure (competing event - death). All eligible patients were followed up to see if they developed CHF or died within 3 years after index AF.
Statistical analysis
Pearson correlation coefficients of baseline comorbidities and variance inflation factors of comorbidities on predicting 3-year CHF were examined to ensure there are no collinearity between comorbidities adjusted for in the study. A Cox proportional hazard model was used to examine the effect of comorbidities in incident AF patients on 3-year heart failure with death as a competing risk. ZPH function test validated proportional hazard assumption, linearity of the continuous variable age in the model was examined by testing addition of its non-linear term age,2 and no significant departure from linearity in the effect of age was observed. Interactions of age and other variables were also not significant.
We employed a Fine and Gray competing risk plot analysis to predict time to incident CHF and probabilities of 3-year incident heart failure in incident AF patients. In this case a competing risks analysis is appropriate to account for censoring which happens when CHF is not observed before the end of study timeline, loss to follow-up, or competing events like dying occurs before CHF can be observed. Like Marianne Huebner et al.18 suggest, in the case a patient dies before a diagnosis of 3-year heart failure can be made, “the censoring assumption that censored observations have the same CHF hazard as those at-risk is not fulfilled, so competing outcomes may need to be considered”.18 The competing risk model, therefore, helped account for dying from old age since our study utilizes CMS data with patients 65-years and older, especially since older patients may be at a higher risk for dying from natural causes before experiencing 3-year heart failure compared to younger patients. Competing risk model hence gives us more accurate estimates for cumulative incidences where competing risks are present19 and are closer to real world probabilities of CHF. All analysis was done using SAS statistical software version 9.4.20
The study comprised of 965,555 patients with incident AF, of which 49.4% were males. 91.3% were White non-Hispanic and 4.7% were Black. Amongst these patients 44.1% had hypertension, 17.9% had CAD, 8.4% had COPD, 18.2% had DM, 4.8% had PAD, 1.1% had an history of MI, 1.1% chronically used steroids, and 2.2% had cardiomyopathy (Table 1).
|
Number of Patients n=965555 (SD, %) |
|||||
|
Male |
476803 (49.38%) |
||||
|
Age(years) |
78.58 ± 8.11 |
||||
|
Race |
|||||
|
White non-Hispanic |
881440 (91.29%) |
||||
|
Black non-Hispanic |
45271 (4.69%) |
||||
|
Asian or Pacific Islander |
11236 (1.16%) |
||||
|
Hispanic or Latino |
10258 (1.06%) |
||||
|
American Indian or Alaska Native |
3315 (0.34%) |
||||
|
Other |
9129 (0.95%) |
||||
|
Unknown |
4906 (0.51%) |
||||
|
Hypertension |
425825 (44.10%) |
||||
|
Coronary Artery Disease |
172813 (17.90%) |
||||
|
COPDa |
81034 (8.39%) |
||||
|
Diabetes Mellitus |
176008 (18.23%) |
||||
|
Peripheral Artery Disease |
47199 (4.89%) |
||||
|
Myocardial Infarction |
10288 (1.07%) |
||||
|
Chronic steroid use |
10729 (1.11%) |
||||
|
Cardiomyopathy |
21644 (2.24%) |
||||
Table 1 Baseline characteristics of patients with incident atrial fibrillation
a,Chronic obstructive pulmonary disease; SD, standard deviation, %, percentage
Following incident AF, males have a 1.8% (HR 0.982, CI 0.974 to 0.990, P<0.001) lower 3-year risk of heart failure compared to females. Presence of hypertension significantly lowers 3-year risk of heart failure by 6.5% (HR 0.935, CI 0.927-0.943, P<0.001).
With every 1-year increase in age of incident AF patients, 3-year risk of CHF increases by 2.6% (HR 1.026, CI 1.026-1.027, P<0.001), presence of coronary artery disease increases risk of incident CHF by 22.1% (HR 1.221, CI 1.208-1.234, P<0.001), COPD by 30.2% (HR 1.302, CI 1.285-1.320, P<0.001), diabetes mellitus by 31.9% (HR 1.319, CI 1.305-1.332, P<0.001), peripheral artery disease by 15.2% (HR 1.152, CI 1.132-1.172, P<0.001), and cardiomyopathy by 103.6% (HR 2.063, CI 2.021-2.105, P<0.001). Blacks have 6.9% higher 3-year risk of CHF after incident AF relative to Whites (HR 1.069, CI 1.050-1.089, P<0.001) (Table 2).
|
Hazard Ratio |
P-Value |
||||
|
(95% CI) |
|||||
|
Male |
0.982 (0.974-0.990) |
<.0001 |
|||
|
Age |
1.026 (1.026-1.027) |
<.0001 |
|||
|
Race (reference=White non-Hispanic) |
|||||
|
Black non-Hispanic |
1.069 (1.050-1.089) |
<.0001 |
|||
|
Asian or Pacific Islander |
0.866 (0.833-0.901) |
<.0001 |
|||
|
Hispanic or Latino |
0.947 (0.911-0.985) |
0.0063 |
|||
|
American Indian or Alaska Native |
1.145 (1.074-1.221) |
<.0001 |
|||
|
Other |
0.860 (0.823-0.899) |
<.0001 |
|||
|
Unknown |
0.762 (0.710-0.817) |
<.0001 |
|||
|
Hypertension |
0.935 (0.927-0.943) |
<.0001 |
|||
|
Coronary rtery Disease |
1.221 (1.208-1.234) |
<.0001 |
|||
|
COPDa |
1.302 (1.285-1.320) |
<.0001 |
|||
|
Diabetes Mellitus |
1.319 (1.305-1.332) |
<.0001 |
|||
|
Peripheral Artery Disease |
1.152 (1.132-1.172) |
<.0001 |
|||
|
Myocardial Infarction |
0.993 (0.958-1.028) |
0.0828 |
|||
|
Chronic Steroid Use |
1.021 (0.983-1.059) |
0.282 |
|||
|
Cardiomyopathy |
2.063 (2.021-2.105) |
<.0001 |
|||
Table 2 Predictors of 3-year incident congestive heart failure in patients with incident atrial fibrillation
a,chronic obstructive pulmonary disease; CI, confidence interval
Overall, after accounting for patient demographics, comorbidities, and death as a competing risk we found that 23.5% of persons with incident AF develop CHF within 3 years of AF diagnosis, 17.5% within the first 2 years and 10% within the first year (Figure 2). The rate of increase in risk of CHF appears to be highest within the first year of AF diagnosis (Figure 2).
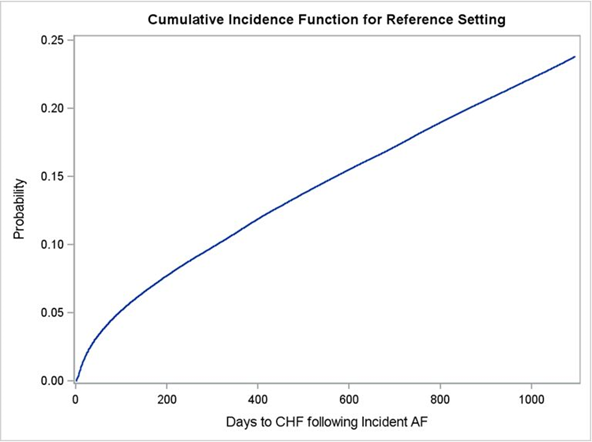
Figure 2 Adjusted cumulative incidence of congestive heart failure within 3 years following incident atrial fibrillation with death as a competing risk.
CHF, congestive heart failure; AF, atrial fibrillation; Reference, a 78.5-year-old white woman with incident AF and no history of hypertension, coronary artery disease, COPD, diabetes mellitus, peripheral artery disease, myocardial infarction, chronic steroid use, and cardiomyopathy
Management of AF and prevention of subsequent cardiovascular events following incident AF remains a challenge. While AF can result in several adverse events, CHF is both the most common non-fatal and cardiovascular event following incident AF, which occurs at more than double the rate of the second most common cardiovascular event- stroke.21 When CHF is developed in incident AF patients, there is an associated 83% increase in hazard rate for all-cause mortality (HR 1.83, CI 1.37-2.45, p<0.0001), 187% increase in cardiovascular mortality (HR 2.87, CI 1.70-4.85, p<0.0001), and 390% increase in myocardial infarction (HR 4.90, CI 2.29-10.50, p<0.0001).22
Our study to our knowledge uses the largest national sample of the US Medicare patient population (study cohort comprises of 965,555 subjects with incident AF) to allow for more precise effect estimate to better understand risk and time to CHF in incident AF patients. We found that the presence of the following comorbidities in patients with incident AF increased the risk of developing CHF: coronary artery disease, COPD, diabetes mellitus, peripheral artery disease, and cardiomyopathy. Likewise, being female, of older age, or Black non-Hispanic relative to white non-Hispanic increases the risk of incident CHF. While some of these findings are consistent with current literature, it is important to note the added findings (not described in prior literature to our knowledge) that in incident AF patients, concurrent COPD increases the 3-year risk of CHF by 30% (HR 1.302, CI 1.285-1.320, P<0.001) while PAD increases the 3-year risk of CHF by 15.2% (HR 1.152, CI 1.132-1.172, P<0.001).
It is therefore paramount that when patients with incident AF are seen with the above comorbidities, closer attention is paid to managing these comorbidities in addition to treating AF in order to reduce the risk of developing CHF. For example, better control of blood sugar in diabetics and treatment of coronary and peripheral artery disease may potentially help mitigate these risk factors for developing CHF in patients with incident AF.
We also found counter-intuitively, that the presence of hypertension in patients with incident AF reduced their risk of developing 3-year CHF. Our finding with hypertension was consistent with the findings of Per Wandell et al.23 who found hypertension lowered risk of CHF in women following incident AF.23 We attribute this to the fact that hypertensive patients who are at least 65 years old like in our study are likely to already be on anti-hypertensive medications and common anti-hypertensives like beta blockers, ACE inhibitors, and angiotensin receptor blockers, have all been shown to reduce the risk of congestive CHF.24
We found that after adjusting for comorbidities, age and other demographics with death as a competing event, risk of developing CHF following incident AF continues to increase over time. For a 78.5-year-old white non-Hispanic woman, cumulative incidence of CHF within 1 year post incident AF is 10%, 17.5% within 2 years, and 23.5% within 3 years. Hence, we recommend future studies to focus on prevention of CHF in patients with incident AF and for clinicians to stay up to date with evidence when assessing potential risk of developing subsequent CHF in each individual patient newly diagnosed with AF depending on what other comorbidities they present with.
Limitations
While association of AF with CHF can be shown in our study, causality however cannot be implied from observational study designs. Also, treatment and assessment of severity of AF using either the Canadian Cardiovascular Society Severity in AF (CCS-SAF) scale or European Heart Rhythm Association (EHRA) score of AF could not be ascertained from using claims data and was not accounted for (a limitation of the data source). We recommend replicating the study using electronic health records to account for this.
AF remains a significant risk factor for CHF, and several other concurrent comorbidities affect risk of new onset CHF in incident AF patients differently. Hence, it is important for treatment and follow up of incident AF patients to be individualized based on their comorbidities to reduce risk of CHF. Also, since the cumulative risk of incident CHF in incident AF patients increase over time with the sharpest rate of increase in the first few years, we recommend following up as well as educating patients on early signs of CHF so they can present early if needed, especially within the first year of incident AF diagnosis.
The author declared that there are no conflicts of interest.
None.
None.

©2023 Togun, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
2 7